One of the top challenges in green organic synthesis and process chemistry is an atom-economical amide synthesis.
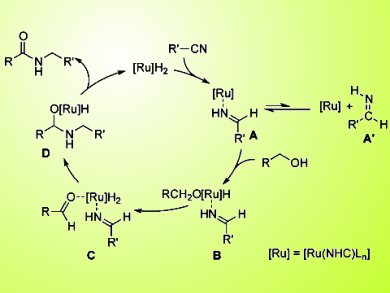
Soon Hyeok Hong, Center for Nanoparticle Research, Institute for Basic Science (IBS), Seoul, Republic of Korea, and colleagues report the first catalytic, single-step, and redox-neutral transformation of alcohols and nitriles into amide with 100 % atom economy. The amide C−N bond is efficiently formed between the nitrogen atom of nitrile and the α-carbon of alcohol. The reaction is catalyzed by an N-heterocyclic carbene-based ruthenium. No byproducts are produced.
A utility of the reaction was demonstrated by synthesizing 13C or 15N isotope-labeled amides without involvement of any separate reduction and oxidation step.
- Ruthenium-Catalyzed Redox-Neutral and Single-Step Amide Synthesis from Alcohol and Nitrile with Complete Atom Economy,
Byungjoon Kang, Zhenqian Fu, Soon Hyeok Hong,
J. Am. Chem. Soc. 2013.
DOI: 10.1021/ja404695t