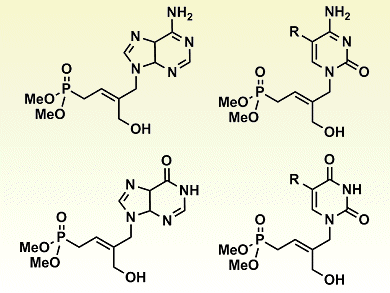
Acyclic nucleoside phosphonates (ANPs) are important antivirals used to treat infections such as hepatitis B and HIV. As a result, the synthesis and biological evaluation of numerous ANPs have been investigated.
After previously reporting a new ANP family that contains a disubstituted trans double bond, Luigi A. Agrofoglio and colleagues, Université d’Orléans, France, and University of St. Andrews, UK, wanted to extend their investigations to trisubstituted derivatives. The synthesis of trisubstituted ANPs by means of standard cross-metathesis ruthenium (Grubbs “second generation”) catalysis proved difficult. Likely owing to the congested double bonds present in one of the starting materials, the use of high catalyst loadings and elevated reaction temperatures gave only poor results with only 4 % conversion. However, when reaction samples were treated with ultrasound, very good conversions rates of up to 93 % could be obtained under mild conditions, that is, 55°C and 15 mol% loading.
Subsequent onward reactions, a regioselective deacetylation with enzyme Candida antarctica lipase B followed by Mitsunobu coupling, gave a range of trisubstituted ANPs (pictured). Antiviral evaluation of the free acid forms of these ANPs is now expected.
- The Preparation of Trisubstituted Alkenyl Nucleoside Phosphonates under Ultrasound-Assisted Olefin Cross-Metathesis,
Ozkan Sari, Manabu Hamada, Vincent Roy, Steven P. Nolan, Luigi A. Agrofoglio,
Org. Lett. 2013.
DOI: 10.1021/ol401922r