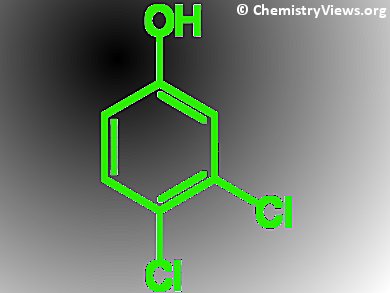
Gautam R. Desiraju and Arijit Mukherjee, Indian Institute of Science, Bangalore, have investigated the crystal structure of 3,4-dichlorophenol. It crystallizes with a tetragonal symmetry, but uniquely there are two distinct types of non-bonding interactions between the chlorine atoms relative to their attached carbon atoms: In type I the two angles are the same, in type II they are approx. 90 °. Usually, only one type of chlorine-to-chlorine non-bonding interaction occurs in any given compound because all of the chlorine atoms are equivalent, sharing the same surroundings. In 3,4-dichlorophenol the interactions between the O atom of the phenolic OH-group and neighboring H atoms produce two different environments allowing both types of Cl…Cl interactions to form.
Additional variable temperature crystallography experiments with analogues of 3,4-dichlorophenol, containing either one or two chlorine atoms substituted for bromine, show that distances between the halogen atoms increase with increasing temperature. This effect is substantially greater in type II contacts than in type I. This might be due to the stronger type II interactions and thus their electrostatic nature.
The type II interaction between two bromine atoms is stronger than that observed in chlorine-only compounds. This makes crystals of 4-bromo-3-chlorophenole more elastic than 3,4-dichlorophenol.
For the first time it has been shown that plastic and elastic deformation in molecular crystals can be explained on the basis of halogen bonding. According to the authors, their finding could lead to elastic, functional materials based on materials with different mixes of carbon-halogen bonds.
 Halogen bonds in some dihalogenated phenols: applications to crystal engineering,
Halogen bonds in some dihalogenated phenols: applications to crystal engineering,
A. Mukherjee, G. R. Desiraju,
IUCrJ 2014, 1, 49–60.
DOI: 10.1107/S2052252513025657