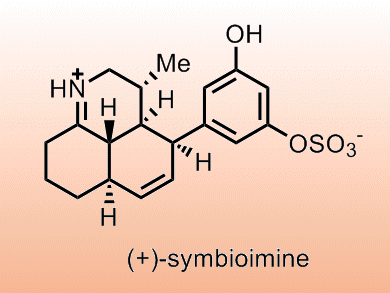
The originally proposed synthesis of (+)-symbioimine involved a tandem diastereoselective exo intramolecular Diels−Alder (IMDA) cycloaddition/imine condensation of the (E)-enone. This approach was not developed due to:
- literature precedents indicating that IMDA cycloadditions proceed preferentially through an endo rather than exo transition state
- the nonconstrained exocyclic C-2 stereocenter controlling the π-facial selectivity for the cycloaddition.
Jason Chruma and co-workers, University of Virginia, USA, have revisited this approach and show that the exo-IMDA cyclization fails due to the all-E triene framework, but the desired cyclization may be achieved through an endo transition state. They note the need to constrain the Z-enone to prevent isomerization and that a single nonconstrained exocyclic stereocenter can control π-facial selectivity in this type of cyclization.
Using this methodology the team formed a range of synthetic precursors to epimeric analogues of (+)-symbioimine.
- Exploring the Original Proposed Biosynthesis of (+)-Symbioimine: Remote Exocyclic Stereocontrol in a Type I IMDA Reaction
J. Burke, M. Sabat, D. Iovan, W. Myers, J. Chruma
Org. Lett. 2010, 12.
DOI: 10.1021/ol101141a
Original paper reporting the isolation and proposed synthesis:
- Symbioimine Exhibiting Inhibitory Effect of Osteoclast Differentiation, from the Symbiotic Marine Dinoflagellate Symbiodinium sp.
M. Kita, M. Kondo, T. Koyama, K. Yamada, T. Matsumoto, K.-H. Lee, J.-T. Woo, D. Uemura
J. Am. Chem. Soc. 2004, 125, 4794.
DOI: 10.1021/ja049277f