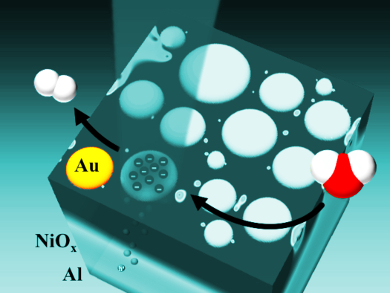
Plasmons are high-energy states or hot electrons that can drive chemical reactions. However, they decay extremely quickly and release their energy as waste heat. To use this energy, hot electrons have to be separated from their corresponding low energy states, the electron holes. Currently, this is done by driving the hot electrons over an energy barrier, the so called Schottky barrier, that acts like a one-way valve. However, this approach is inefficient.
Isabell Thomann and colleagues, Rice University, Houston, TX, USA, used a three-layer setup as a plasmonic photoelectrode architecture for solar-to-energy conversion. The bottom layer is a thin sheet of aluminum, the middle layer a thin coating of transparent nickel oxide, and the top layer is a collection of plasmonic, puck-shaped gold nanoparticles with a diameter of 10–30 nm. The nanoparticles convert the light energy into hot electrons. The aluminum attracts the resulting electron holes and the nickel-oxide allows these to pass while acting as a barrier to the hot electrons. These stay on the gold.
When the material is covered with water, the gold nanoparticles act as a catalyst for water splitting. The cathodic photocurrents show very good performance, even though the system does not use platinum as a co-catalyst and uses much smaller catalytic areas and a small amount of gold compared to conventional electrodes. The team has thus developed an efficient process. However, better understanding is needed to further improve the quantum efficiency before hot charge carriers can widely be used in catalysis.
- Direct Plasmon-Driven Photoelectrocatalysis,
Hossein Robatjazi, Shah Mohammad Bahauddin, Chloe Doiron, Isabell Thomann,
Nano Lett. 2015, 15, 6155–6161.
DOI: 10.1021/acs.nanolett.5b02453