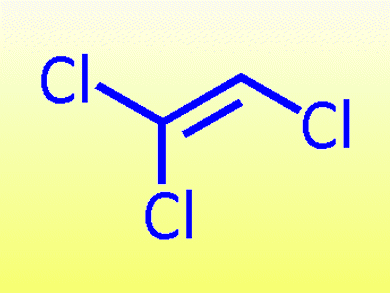
Tri- and tetrasubstituted alkenes are ubiquitous and found in a range of biologically active compounds such as the anticancer drug (Z)-tamoxifen. Laina Geary and Philip Hultin, University of Manitoba, Canada, report a modular approach to their synthesis starting from trichloroethylene (TCE).
The approach uses the inherently different selectivities of each of the three chloro groups to control and direct the functionalization. First, (E)-1,2-dichlorovinyl ether is formed by substitution with phenol. The C1–Cl can then undergo cross-coupling with a variety of organometallic reagents catalyzed by palladium. Next, C2–Cl can be functionalized in a similar manner, leaving the C2–H free to be deprotonated and quenched with an electrophile.
This method efficiently substitutes alkyl, alkenyl, alkynyl, or (hetero)aryl groups at the C1 and C2 positions of TCE.
- Palladium-Catalyzed Modular Assembly of Electron-Rich Alkenes, Dienes, Trienes, and Enynes from (E)-1,2-Dichlorovinyl Phenyl Ether
L. M. Geary, P. G. Hultin,
J. Org. Chem. 2010, 75.
DOI: 10.1021/jo1014678

![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)

