Chiral carbon nanostructures show promise for polarization-enabled optoelectronic devices and photocatalysts, but until now, chiral graphene nanoribbons have only been formed by chance and found as individual particles.
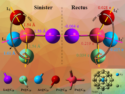
Angela Violi and Nicholas Kotov, University of Michigan, Ann Arbor, USA, and colleagues found that bonding cysteine to the edges of graphene quantum dots caused the dots to buckle. Intermolecular interactions among the L-cysteine or D-cysteine ligands caused the graphene sheets to form right-handed or left-handed helices, respectively. The helices exhibit chiroptical activity at wavelengths longer than those for the cysteine ligands, indicating that the ligands perturb the electronic states of the graphene.
Modeling studies showed that the chiral electronic states of the edge ligands on smaller graphene quantum dots are more likely to be hybridized, because the electronic states of the cysteine groups and the small graphene sheets differ less than for larger quantum dots. At sufficiently high temperatures, the buckled sheets can change conformation. This happens more easily for large graphene quantum dots, which form racemic mixtures, regardless of the chirality of the ligands.
- Chiral Graphene Quantum Dots,
Nozomu Suzuki, Yichun Wang, Paolo Elvati, Zhi-Bei Qu, Kyoungwon Kim, Shuang Jiang, Elizabeth Baumeister, Jaewook Lee, Bongjun Yeom, Joong Hwan Bahng, Jaebeom Lee, Angela Violi, Nicholas A. Kotov,
ACS Nano 2016.
DOI: 10.1021/acsnano.5b06369