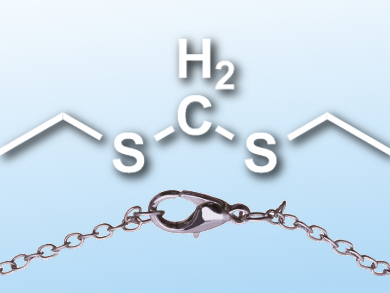
Disulfide bonds are important for the three-dimensional structure of peptides and proteins. However, they are unstable towards reducing agents and nucleophiles. This can be a problem, for example, when peptides are used as pharmaceutical agents, since it limits their stability. Replacing the disulfide groups with more stable bridges can distort the peptides’ geometry and decrease their activity.
Christopher M. B. K. Kourra and Nicolai Crame, Ecole Polytechnique Fédérale de Lausanne (EPFL), Switzerland, have developed a procedure that converts disulfide bridges in peptides directly into the more stable methylene thioacetals (pictured). The two-step process involves a reduction of the disulfide groups to thiols, using tris‐(2‐carboxyethyl)phosphine (TCEP), and a methylene insertion, using diiodomethane (CH2I2) as a methylene source and triethylamine (NEt3) as a base. The reaction features mild, biocompatible conditions (aqueous solution, mild pH, and ambient temperature) and tolerates unprotected amino acids.
The team tested the method on a range of bioactive peptides, including octreotide (a growth hormone inhibitor), oxytocin (used to contract uterine tissue), and vasopressin (used to increase blood pressure). The “reinforced” methylene thioacetal derivatives were obtained in good to excellent yields. The modified peptides showed improved stability under reductive conditions and at elevated temperatures.
The methylene groups are a very small linker and cause only a slight structural disturbance. The modified peptides maintained binding affinities and functional activities close to those of the natural peptides. According to the researchers, the method could have an impact on the medicinal application of bioactive peptides.
- Converting Disulfide Bridges in Native Peptides to Stable Methylene Thioacetals,
Christopher M. B. K. Kourra, Nicolai Cramer,
Chem. Sci. 2016.
DOI: 10.1039/c6sc02285e