The epoxidation of allenes, compounds with two adjacent C=C double bonds, gives allene oxides. While these species are very reactive, there have been stable allene oxides reported as early as 1968. However, no stable allene oxides with bromine substituents and no crystal structure of an allene oxide had ever been obtained.
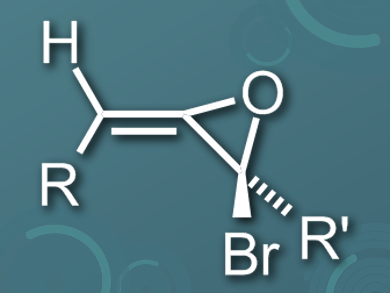
D. Christopher Braddock and colleagues, Imperial College London, UK, have synthesized and characterized the first stable bromoallene oxides. The team prepared a range of adamantyl-substituted bromoallenes by lithiating terminal alkenes and subsequently coupling them to adamantane carboxaldehyde. Then they performed an epoxidation using dimethyldioxirane (DMDO) to give E-1-bromoallene oxides as single diastereomers (pictured).
One of the prepared compounds, featuring one adamantyl and one tert-butyl substituent, was a crystalline solid. The researchers were able to characterize this compound, the first crystalline allene oxide of any kind, using X-ray crystallography.
- Stable bromoallene oxides,
D. Christopher Braddock, Areeb Mahtey, Henry S. Rzepa, Andrew J. P. White,
Chem. Commun. 2016.
DOI: 10.1039/C6CC06395K

![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)

