The oxidation of primary alcohols to the corresponding carboxylic acids can be found in every organic chemistry textbook. Nevertheless, there are still new approaches, such as dehydrogenation reactions which release molecular hydrogen. Such reactions have been used for the synthesis of amides, imines, esters, and acetals from alcohols. However, the corresponding syntheses of carboxylic acids have been more difficult to achieve.
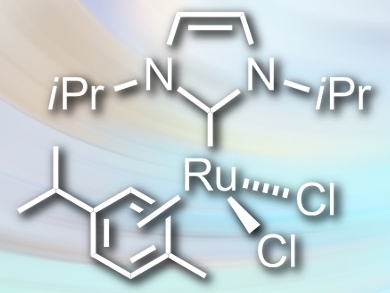
Robert Madsen and colleagues, Technical University of Denmark, Lyngby, have developed a method for the dehydrogenative synthesis of carboxylic acids from primary alcohols. The team reacted the alcohols with potassium hydroxide in the presence of the ruthenium catalyst [RuCl2(NHCiPr)(p-cymene)] (pictured) in toluene. The reaction gives the corresponding carboxylic acids in high yields, with molecular hydrogen as the only byproduct.
The products were isolated by precipitation of the potassium salts, followed by treatment with aqueous hydrochloric acid, and extraction with ethyl acetate. The isolated acids did not require further purification by chromatography, distillation, or recrystallization. According to the researchers, the results show the potential of the ruthenium catalyst for the development of new dehydrogenative transformations of alcohols.
- Dehydrogenative Synthesis of Carboxylic Acids from Primary Alcohols and Hydroxide Catalyzed by a Ruthenium N-Heterocyclic Carbene Complex,
Carola Santilli, Ilya S. Makarov, Peter Fristrup, Robert Madsen,
J. Org. Chem. 2016.
DOI: 10.1021/acs.joc.6b02105