Functionalized porphyrins play key roles in many biological processes, such as photosynthesis. Due to their photochemical and coordinative properties, they are also interesting targets for synthetic chemistry with a wide range of potential applications. However, the selective functionalization of porphyrins to fine-tune their molecular properties is still a challenging task. One approach to this is the introduction of phosphonate substituents, which provide Lewis-basic sites and can be hydrolyzed to the corresponding acids.
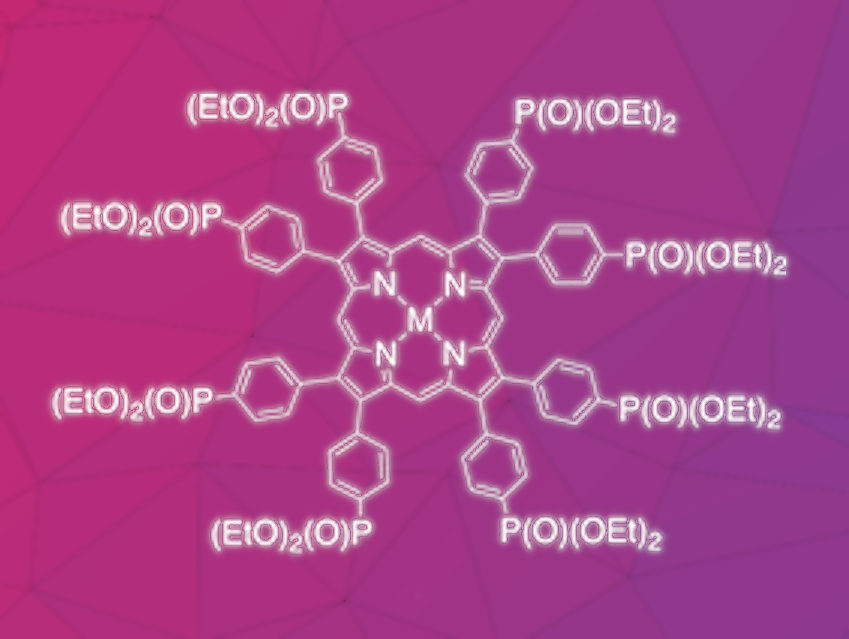
Elizaveta V. Ermakova, Russian Academy of Sciences, Moscow, Karl M. Kadish, University of Houston, TX, USA, Alla Bessmertnykh-Lemeune, Université Bourgogne Franche-Comté, Dijon, France, and École normale supérieure de Lyon, France, and colleagues have developed a porphyrin ligand substituted with eight (diethoxyphosphoryl)phenyl groups in β-positions of the macrocycle (general metal complex pictured).
The ligand was prepared by a stepwise synthesis route starting with the synthesis of a substituted pyrrole building block. 4,4′-dibromobenzil was condensed with dimethyl-N-acetyliminodiacetate, followed by decarboxylation to give a pyrrole derivative. The phosphonate group was then introduced via a Pd-catalyzed Hirao coupling. The porphyrin ring was constructed by a condensation with formaldehyde, followed by oxidation with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ), which provided the target ligand in 73 % yield.
The new ligand readily forms complexes with different bivalent metal cations (Ni, Cu, Zn, Cd) in over 90 % yield. In UV-Vis-spectroscopic measurements, evidence for the formation of dimers was obtained for the Zn complex. Single-crystal X-ray diffraction of the Zn complex showed the presence of self-assembled dimers in the solid state. These are stabilized by a bridging water molecule that coordinates the Zn center of one complex and forms a hydrogen bond with the phosphoryl group of a second complex. According to the researchers, these self-assembled dimers can be regarded as new model systems for naturally occurring photosynthetic antenna systems.
- Synthesis and Self-Assembly of β-Octa[(4-Diethoxyphosphoryl)phenyl]porphyrins,
Anton V. Shukaev, Elizaveta V. Ermakova, Yuanyuan Fang, Karl M. Kadish, Sergey E. Nefedov, Victor A. Tafeenko, Julien Michalak, Alla Bessmertnykh-Lemeune,
Inorg. Chem. 2023.
https://doi.org/10.1021/acs.inorgchem.2c03466