Oxidative N-heterocyclic carbene (NHC) catalysis is a useful type of carbene catalysis. However, it is traditionally limited to closed-shell species as reactive intermediates because the process usually involves the transfer of two electrons. In contrast, NHC radical catalysis involves the catalytic generation of active radical reactants and can be effective for challenging transformations. However, it often requires matching the redox potential between substrates and the NHC catalytic system.
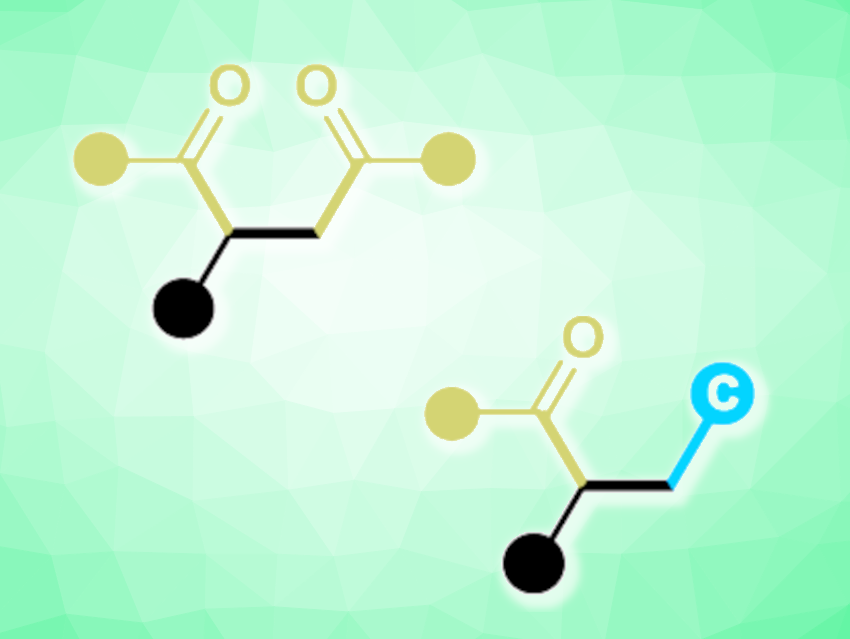
Jun-Long Li, Chengdu University, China, and colleagues have developed an unprecedented oxidative radical NHC catalytic system for activating inert C–H bonds in simple substrates (pictured below). Commercially available thiazolium-based NHCs were combined with tert-butyl naphthalene-2-carboperoxoate that functions as a single-electron oxidant to trigger a sequential single-electron oxidation and intermolecular hydrogen-atom transfer (HAT) reaction. Using this approach, the team achieved efficient syntheses of difunctionalized olefins (general structures pictured below).
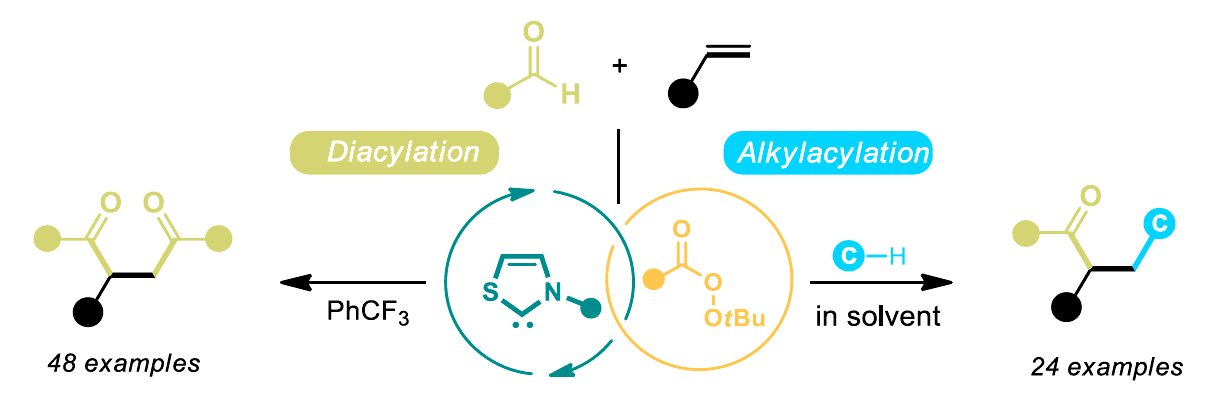
A variety of 1,4-diketone products (pictured in green on the left) were produced in high yields, and a diverse range of alkylacylated olefins (pictured in green and blue on the right) were obtained when solvents such as dichloromethane, chloroform, tetrahydrofuran, and 1,4-dioxane were used. According to the researchers, this organocatalytic method could also be relevant for the late-stage modification of drug skeletons.
- Oxidative Radical NHC Catalysis: Divergent Difunctionalization of Olefins through Intermolecular Hydrogen Atom Transfer,
Qing-Zhu Li, Yan-Qing Liu, Xin-Xin Kou, Wen-Lin Zou, Peng Xiang, Jin-Dun Xing, Ting Qi, Xiang Zhang, Jun-Long Li,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202207824