Have you ever wondered why glow sticks (see Fig. 1) are glowing after you have bent them? Or why those plastic stars are still glowing at night after the lights have been switched off? You may not have. However, whether you have asked yourself those questions or not, the following text will hopefully provide you with answers and explanations of the phenomena of glowing bracelets, shining jellyfish, black light, and many more.
|
|
|
Figure 1. Glow sticks. |
Luminescence
In general, the glow that occurs in all the aforementioned phenomena is called luminescence. Luminescence is energy released by a substance in the form of light [1,2]. Several types of luminescence can be differentiated:
- One example is chemiluminescence. During some chemical reactions, energy is released as light. This occurs after bending a glow stick. It is also the reason for the glowing of animals like jellyfish or some microorganisms. In this case, it is called bioluminescence [1].
- Another kind is triboluminescence. This can be observed when a self-adhesive envelope is opened in complete darkness or when adhesive tape is unrolled in a dark room. In doing so, mechanical energy is put into the system and serves as an activator for the glow [3].
- Probably the most familiar type of luminescence is photoluminescence. Here, energy is provided by electromagnetic radiation, for instance through sunlight or an ultraviolet lamp, as in some discotheques. This causes phenomena like the ongoing glow of plastic stars or the extreme brightness of white clothes under black light. One can differentiate fluorescence and phosphorescence, which will be explained below [1].
What is an Electronically Excited State?
Generally, all kinds of luminescence are based on so-called photo-physical processes[4]. Usually, molecules themselves are described as fluorescent. This is the case with fluorescent dyes like fluorescein or curcumin [1,5]. However, to explain photo-physical processes, one has to take a closer look at an even smaller level than the molecular one.
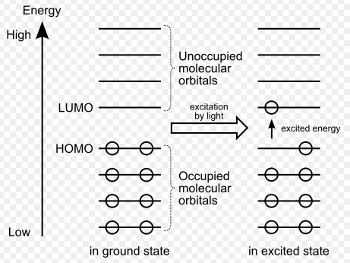
Atoms of different elements have a different number of electrons distributed into several shells and orbitals. Electrons are a type of elementary particle. Electronic transitions are responsible for luminescence [6,7]. When the system absorbs energy, electrons are excited and are lifted into a higher energetic state. Before excitation, in the ground state, some of the electrons are in the so-called HOMO (Highest Occupied Molecular Orbital). After they reach an excited state, they are in the LUMO (Lowest Unoccupied Molecular Orbital) [1] (see Fig. 2). How this works exactly will be explained using photoluminescence as a specific example.
|
|
|
Figure 2. Electronic excitation. |
Different energetic states of an atom or molecule are known as “energy levels”. Depending on the molecule and atom, the electrons can only occupy discrete energy levels since the energy is quantized, which means, energy can only be absorbed and emitted in certain amounts [8]. The difference between two levels can be calculated with equation 1 (where E2 is the higher energy level and E1 the lower one).
(1) ΔE = E2–E1
Photons, particles of which electromagnetic radiation or light consists, have to have a certain energy value to be able to excite electrons. The energy of a photon can be calculated with equation 2, where h is the Planck constant and ν is the frequency of the light.
(2) Ephoton = hν
The necessary excitation energy for the electrons equals the difference between the energy levels. Only light with a certain energy, and accordingly with a certain frequency and wavelength, is capable of exciting electrons [3]. By equalizing equations 1 and 2, and with the help of equation 3 (where c stands for the speed of light), the necessary frequency and wavelength can be calculated (see eq. 4) [9]. In many cases, UV-radiation is used for excitation.
(3) λ = c/ν
(4) ΔE = Ephoton ⇔ E2 – E1 = hν
ν = (E2 – E1)/h
λ = hc/(E2 – E1)
Deactivation of Electronically Excited States
Such electronically excited states are unstable. Electrons drop back to their ground states. At the same time, the excitation energy is released again. One distinguishes between radiative and non-radiative decay processes. Most of the time, the decay is non-radiative, for example through vibrational relaxation, quenching with surrounding molecules, or internal conversion (IC) [6,7,10]. These processes will be explained in detail later.
Sometimes, a radiative decay can occur in form of fluorescence and phosphorescence. The energy is emitted as electromagnetic radiation or photons. The emitted light has a longer wavelength and a lower energy than the absorbed light because a part of the energy has already been released in a non-radiative decay process [10]. This is the reason that an emission in the visible spectrum can be achieved by excitation with non-visible UV-radiation. This shift towards a longer wavelength is called Stokes shift [11].
Comparison: Fluorescence vs. Phosphorescence
Both fluorescence and phosphorescence are spontaneous emissions of electromagnetic radiation. The difference is that the glow of fluorescence stops right after the source of excitatory radiation is switched off, whereas for phosphorescence, an afterglow with durations of fractions of a second up to hours can occur [6,7].
To compare the photo-physical processes behind both phenomena, there are some facts about electrons that are helpful for understanding: Electrons are particles that have a so-called spin and a spin quantum number. This can have two different values, namely either +1/2 or –1/2 [6]. This number is a property that we actually cannot imagine or describe easily. It is often compared with a spinning top, either spinning in a clockwise or anti-clockwise direction. However, this description is neither mathematically nor physically quite correct. Two electrons in a single orbital of an atom have antiparallel spin, which is noted as (↑↓) [6,12].
Fluorescence
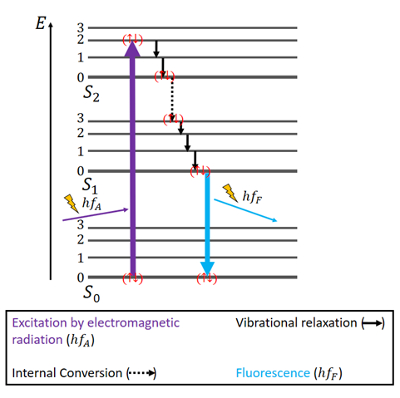
In the Jablonski diagram for fluorescence (see Fig. 3), the singlet spin state S0 is the ground state of the electrons, and S1 and S2 are singlet excited states (the states are only used as an example in this text and do not necessarily apply to certain atoms, molecules, etc.). Within those states, there are several energy levels. The higher the level is, the more energy an electron possesses when being in that level. In the case of singlet states, the electrons have antiparallel spins.
|
|
|
Figure 3. Jablonski diagram for fluorescence. |
The electrons are lifted from the ground state S0, for example, to an energy level of the second excited state S2, when excited by electromagnetic radiation. After excitation stops, the electrons only stay in that excited state for a short period of time (ca. 10–15 s) and then immediately start falling back down into the ground state [6]. In doing so, energy initially can be released to the surroundings by vibrational relaxation. That means thermal energy is released by the motion of the atom or molecule until the lowest level of the second excited state is reached.
The bigger gap between the second and first excited state is overcome by internal conversion. That describes an electronic transition between two states while the spin of electrons is maintained. Now, the electrons can relax further due to more vibrational relaxation until they reach the lowest energy level of the S1 state.
Theoretically, the electrons could relax even further in a non-radiative way until they eventually reach the ground state again. However, it can be the case that the last amount of energy is too large to be released to the surroundings because the surrounding molecules cannot absorb this much energy. Then, fluorescence occurs, which leads to an emission of photons possessing a certain wavelength. The emission lasts only until the electrons are back in the ground state. Since during all those transitions the electron spin is kept the same, they are described as spin-allowed [6,7,10].
Phosphorescence
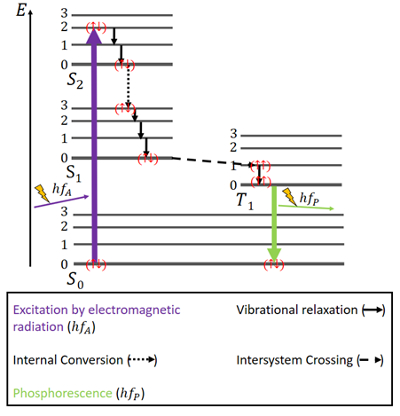
For phosphorescence, things are a bit different (see Fig. 4). There are again an S0 ground state and the two excited states, S1 and S2. Additionally, there is an excited triplet T1 state which lies energetically between the S0 and S1 state. The electrons again have antiparallel spins in the ground state.
|
|
|
Figure 4. Jablonski diagram for phosphorescence. |
Excitation happens in the same way as in fluorescence, namely through electromagnetic radiation. The release of energy through vibrational relaxation and internal conversion while maintaining the same spin is the same here, as well, but only until the S1 state is reached.
Alongside the singlet states, a triplet state exists and so-called intersystem crossing (ISC) can occur since the T1 state is energetically more favorable than the S1 state. This crossing, like internal conversion, is an electronic transition between two excited states. But contrary to internal conversion, ISC is associated with a spin reversal from singlet to triplet. Electrons in the triplet state have parallel spins, which is noted as (↑↑) [6]. This ISC process is described as “spin-forbidden”. It is not completely impossible – due to a phenomenon called “spin-orbit coupling” – however, it is rather unlikely [7].
In the T1 state, non-radiative decay is possible as well. However, a transition between the lowest energy level of the triplet state and the S0 state is not readily possible, because that transition is spin-forbidden, too. Still, it can happen anyway with a small possibility. It causes a rather weak emission of photons because the electron spin has to be reversed again. The energy is trapped in this state for a while and can only be released slowly [6]. After all energy has been released, the electrons are back in the ground state [6,7,10].
Conclusion
The spin-allowed and -forbidden processes serve as explanations for an immediately ceasing glow of fluorescence and for the afterglow of phosphorescence. Phosphorescence usually occurs only with “heavier” molecules since the spin has to be reversed with the help of spin-orbit-coupling. Whether electromagnetic radiation is emitted at all, and with which wavelength, depends on how much energy can be released beforehand by non-radiative decay [6,7]. It also depends on the properties of so-called quenchers that are surrounding molecules and are able to take up larger amounts of energy.
All processes that can lead to an inhibition of radiative decays can cause fluorescence quenching. Examples are non-radiative decay processes, but also the destruction of the fluorescent molecule [10]. The quantum efficiency describes the efficiency of the process and is defined as the ratio of absorbed and emitted photons [13]. This property is different for each substance.
Even though this text focuses on photoluminescence, the photo-physical processes are the same for all types of luminescence [4].
Applications
In addition to products like glow sticks, fluorescence and phosphorescence are used in many other ways. Further examples are guideposts leading to an emergency exit that need no electric supply but glow at night due to phosphorescence. Even plants can be made fluorescent: Spinach can be modified with the help of nanotechnology so that it can detect traces of explosive substances in the groundwater. The leaves contain carbon nanotubes to which nitroaromatics can bond. If they do, a fluorescent signal is released by the plant and can be detected with infrared cameras [14].
The video demonstrates different types of luminescence. On the left-hand side, it shows the fluorescence of the dye curcumin, which is contained in the spice turmeric, under UV light [5,15]. Curcumin is dissolved in alcohol to make the fluorescence visible.
The plastic spider and the compound in the small tube are examples for phosphorescence. Strontium aluminate, which is contained in the tube, is initially excited by UV radiation and eventually emits green light. The cause for this is a doping with elements like europium, which makes the compound usable as a luminescent pigment [15].
Bending the glow stick (at the right-hand side) initiates a chemical reaction between hydrogen peroxide and a dye and phenyl oxalate. Chemiluminescence can be observed.
Video 1. Fluorescence, phosphorescence, and chemiluminescence in comparison.
References
[1] K. Arnold et al., Chemie Oberstufe (in German), Cornelsen Schulverlage, Berlin, 2015, 496-497. ISBN: 978-3-06-011179-4
[2] Lexikon der Physik: Lumineszenz (in German), spektrum.de. (accessed January 25, 2017)
[3] D. Wiechoczek, Wenn Mineralien selber leuchten – Phosphoreszenz, Fluoreszenz und Lumineszenz (in German), chemieunterricht.de 2010. (accessed January 25, 2017)
[4] Lexikon der Physik: Photophysikalische Prozesse (in German), spektrum.de. (accessed January 27, 2017)
[5] D. Wiechoczek, Chemie mit Curry (in German), chemieunterricht.de 2015. (accessed January 27, 2017)
[6] P. W. Atkins, J. de Paula, Kurzlehrbuch Physikalische Chemie (in German), Wiley-VCH, Weinheim, 2008, 853ff., 921ff. ISBN: 978-3-527-31807-0
[7] P. W. Atkins, Physical Chemistry, Oxford University Press, 1994, 591ff.
[8] Quantelung (in German), chemie.de. (accessed January 27, 2017)
[9] Bohr’sche Frequenzbeziehung (in German), chemgapedia.de. (accessed January 25, 2017)
[10] Technische Universität Ilmenau, Praktikum Physikalische Chemie II: Physikalische Chemie/Mikroreaktionstechnik, Versuch Fluoreszenz-Quenching(in German), tu-ilmenau.de. (accessed January 27, 2017)
[11] Stokesverschiebung (in German), chemie.de. (accessed January 25, 2017)
[12] Elektronenspin (in German), chemie.de. (accessed January 27, 2017)
[13] Quantenausbeute (in German), chemie.de. (accessed January 27, 2017)
[14] Spinat erkennt Sprengstoff im Grundwasser (in German), Spiegel Online, November 1, 2016. (accessed January 27, 2017)
[15] D. Weiß, Phosphoreszenz (in German), chemie.uni-jena.de. (accessed January 27, 2017)

Kim Dreier is a student at the Marianne-Weber-Gymnasium (secondary school) in Lemgo, Germany.






Brilliant presentation. I collect fluorescent minerals from Franklin, New Jersey, and I’ve always wondered the mechanism of fluorescence. This article explain it well. But how do activators, like manganese, impart fluorescence?
Great clarity in presenting the topic
I was wondering before I purchase “firefly” earrings that have phosphorescent particles which are inside the glass beads would be safe for my granddaughter who is 7 to wear? I think she would like them but I was concerned about the phosphorescent particles.
Thanks in advance for your help of a concerned grandparent,
Jackie