Alfred Wöhlk was a Danish chemist and pharmacist who lived from 1868 to 1949. On Juli 25, 2018, he would be 150 years old. With his historical detection reaction from 1904, the lactose content of dairy products can be visualized. This is useful because many people are lactose intolerant and avoid dairy products. Countless pharmacy students, but also physicians, laboratory assistants, and recently also chemistry teachers know the Wöhlk test. Despite many efforts, the reaction mechanism is still unknown. Nevertheless, the experiment is colorful, meaningful, and easy to implement.
What Can the Wöhlk Test Be Used For?
Since its discovery in 1904 until the 1960s, the Wöhlk test has mainly been used to detect the reducing disaccharide lactose in urine in medical laboratories. Although there were already a number of wet-chemical detection methods to identify sugars, such as the Fehling or Benedict test, those analyses could only differentiate between reducing and non-reducing sugars. Since both glucose and lactose molecules have an aldehyde group in the open-chain form, the result was positive in both cases. Only the Wöhlk test allowed a safe differentiation between glucose and lactose.
Thus, a relatively harmless milk congestion (lactosuria) could be differentiated from a dangerous gestational diabetes. With lactose, the tested urine develops a significant red color, with glucose, only a yellow dye develops, and with sucrose, the color does not change (see Fig. 1).
 |
|
Figure 1. Wöhlk reaction with different dairy products: lactose produces a salmon red color, while other sugars produce a yellow tint (glucose) or no color (sucrose). © Photo: Ruppersberg |
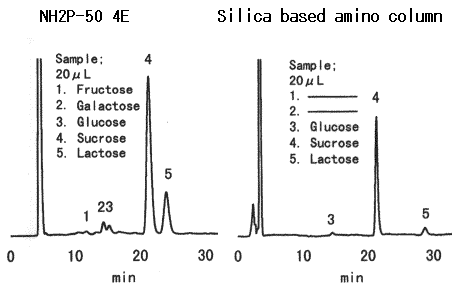
Today, the Wöhlk test is hardly used in the laboratory; modern methods that primarily use chromatography are faster and more accurate (see Fig. 2).
 |
|
Figure 2. Modern separation of sugars in a high-performance liquid chromatography (HPLC) column, here: 20 µL of yogurt (Source: https://www.shodex.de). |
Nevertheless, the Wöhlk test has a very special appeal due to its splendid coloring (see Fig. 3). In addition, it is highly informative for school chemistry classes, because it allows the semi-quantitative analysis of dairy products for their lactose content. In this way, it can be clearly explained in class that not only normal and lactose-free milk differ, but also that kefir, milk-based creamer, and other dairy products have different lactose contents. Students in whose family lactose intolerance occurs should be very interested in their own tests with dairy products, and for others, the Wöhlk test still is an interesting experiment.
 |
|
Figure 3. Wöhlk test tubes in a water bath at 70 °C. Photo: Ruppersberg |
How to Do the Wöhlk Test
1. Use
Different amounts of lactose in dairy products can be detected semi-quantitatively.
2. Preparation
Buy some dairy products in the supermarket, e.g., whole milk (cow’s milk), lactose-free milk, buttermilk, kefir, natural yogurt, dairy-based coffee creamer, and sour cream. These products should be as natural as possible. Adding of colorants or sugars such as fructose and glucose would distort the results of the experiment.
For comparative purposes, you should have lactose, fructose, glucose, galactose, and sucrose as pure substances.
As reagents, you need 1 molar potassium hydroxide solution , best in a dropper bottle, as well as 10 % ammonia solution (by mass)
with a pipette to measure 2 mL.
Equipment: A thermometer to measure 70 °C; a water bath made up of a 1 L beaker filled with approximately 300 mL of water, and a hot plate; test tubes; test tube holder; test tube rack; disposable pipettes; (smartphone) camera.
3. Method
Recordings of all important steps and changes are made for documentation purposes with the (smartphone) camera.
- 300 mL of water are heated to 70 °C in a 1 L beaker.
- 2 mL of each of the different dairy products are pipetted into numbered test tubes with disposable pipettes. To ensure that the outcome is not disturbed, the dairy products must not contain red dyes or either glucose or fructose. In this example, the following products were used: 1) whole milk, 2) lactose-free milk, 3) buttermilk, 4) kefir, 5) natural yogurt, 6) dairy-based coffee creamer, 7) sour cream.
- Depending on the viscosity of the dairy products, the tips of the disposable pipettes might need to be slightly shortened.
- In five more test tubes, 50 mg of each of the following sugars are added and dissolved in 2 mL of water each: lactose, fructose, glucose, galactose, and sucrose (see Fig. 4).
- Subsequently, 2 mL ammonia solution and 3 drops of potassium hydroxide solution are pipetted and added to each test tube. The solutions are carefully but thoroughly shaken so that everything mixes well.
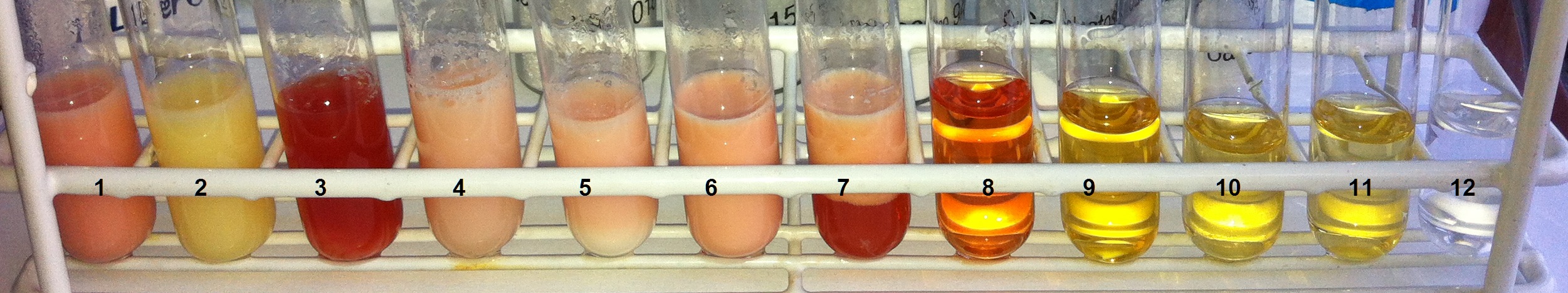
- The test tubes are then placed in the hot water bath and left there for at least 15 minutes until the colors of the samples have developed well. Every 5 minutes, a visual inspection with photo documentation takes place (see Fig. 5).
- After 30 minutes at the latest, all test tubes are removed from the water bath.
 |
|
Figure 4. Test tubes before the Wöhlk test: No. 1–7: Dairy products (left), No. 8–12: sugar solutions (right) [1]. |
 |
|
Figure 5. After the Wöhlk test: From left to right, the test tubes contain 1) whole milk, 2) lactose-free milk, 3) buttermilk, 4) kefir, 5) natural yogurt, 6) coffee creamer, 7) sour cream, 8) lactose, 9) fructose, 10) glucose, 11) galactose, 12) sucrose [1]. |
4. Observation
Depending on the lactose content of the dairy product examined, a different degree of red coloration is visible. Solutions of fructose, glucose, and galactose produce different shades of yellow, while the sucrose solution remains clear and colorless.
5. Note
In some countries, the very similar Fearon’s test is better known. In this case, ammonia is replaced by a methyl ammonium chloride solution [2] (see Tab. 1).
|
Table 1. N-containing compounds in Wöhlk and Fearon’s test. |
|||||||||||||||
|
|||||||||||||||
In addition to that, the Wöhlk test delivers valuable information when performed after the starch cleavage by saliva amylase, where it serves to detect the disaccharide maltose. Maltose is the main product of endohydrolytic cleavage besides glucose, isomaltose, and other compounds. The test shows how much maltose is present. (see Fig. 6)
|
Table 2. Detected molecules in Wöhlk and Fearon’s test. |
|||||||||||||||
|
|||||||||||||||
 |
|
Figure 6. Wöhlk test: The content of maltose or lactose in different solutions is inducated by the intensity of the color. © Photo: Ruppersberg |
Mechanism
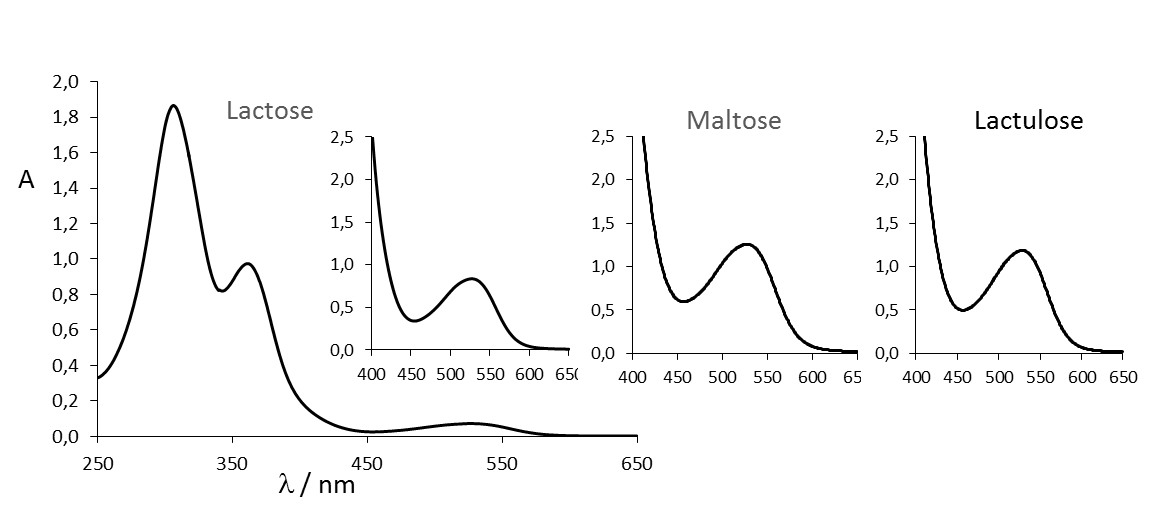
The exact mechanism of the Wöhlk reaction is unknown despite numerous efforts. The structure of the red dye is still a mystery because it is stable only in a strongly alkaline environment and cannot be extracted with known solvents. However, it is now known that the red dye has an absorption maximum at 527 nm (see Fig. 7) and is also formed with other sugars which are analogous to lactose and maltose (e.g., cellobiose and maltotriose, see also Fig. 8).
 |
|
Figure 7. UV/Vis spectra of the “Wöhlk products” of lactose (full spectrum with enlarged detail in the area responsible for the red color), maltose and lactulose (excerpt) [3]. |
The absorption band at 527 nm is so symmetrical and identical for all three sugars (see Fig. 7) that one can assume a uniform component. The sugars cellobiose, maltulose, and maltotriose show a similar absorption spectrum. Even a glucose molecule with a protective group at C4 is sufficient. It also seems clear that the mechanism starts with Lobry de Bruyn–van Ekenstein rearrangements, since the rearrangement products lactulose and maltulose also form the typical salmon red dye with an absorption maximum at 527 nm in the Wöhlk test.
 |
|
Figure 8. Sugar solutions after the Wöhlk test (from left to right: in each tube 50 mg lactose, maltose, cellobiose, lactulose, sucrose, glucose, fructose, galactose, mixture galactose/glucose, according to instructions above. © Photo: Ruppersberg |
Alfred Wöhlk
Alfred Wöhlk was born in Frederikshavn (“the tip of Denmark”) on July 25, 1868, as the eighth child of his parents Clara Wilhelmine Wöhlk, née Knutzen, and Carl Andreas Nicolai Wöhlk. He had ten siblings and studied at the Pharmaceutical School in Copenhagen, Denmark, where he also worked and published in German. In 1910, he became the owner of the Trianglen Pharmacy in Copenhagen (see Fig. 9), which he ran until his death in 1949.
In addition, he also published for the Danmarks Farmaceutiske Selskab (Denmark’s Pharmaceutical Society) and invented the drug Magnyl®, a Danish acetyl salicylic acid variation with magnesium oxide.
 |
|
Figure 9. Alfred Wöhlk (front) and coworkers in the Trianglen pharmacy Copenhagen. © Photo: Poul Schjøtz Nissen; København Apoteket Trianglen |
References
[1] K. Ruppersberg, J. Hain, Wie kann der Lactosegehalt von Milchprodukten im Schulexperiment sichtbar gemacht werden?: Die Wiederentdeckung der Wöhlk-Probe für den Chemieunterricht (in German), CHEMKON 2016, 23, 90–92. https://doi.org/10.1002/ckon.201610272
[2] W. R. Fearon, The detection of lactose and maltose by means of methylamine, Analyst 1942, 67, 130–132. https://doi.org/10.1039/AN9426700130
[3] K. Ruppersberg, J. Hain, P. Mischnick, Auf der Spur der roten Farbe: Ein historischer Lactosenachweis wiederentdeckt (in German), CHEMKON 2017, 24, 302. https://doi.org/10.1002/ckon.201790012
Further Reading
- K. Ruppersberg, J. Hain, Die Wiederentdeckung der Wöhlk-Probe und der geheimnisvolle lachsrote Farbstoff (in German), Chem. Unserer Zeit 2017, 51, 106–11. https://doi.org/10.1002/ciuz.201600744
- K. Ruppersberg, Dem Milchzucker auf der Spur – eine europäische Detektivgeschichte (in German), Praxis der Naturwissenschaften – Chemie in der Schule 2016, 65, 30–33.
- K. Ruppersberg, Stärkeverdauung durch Speichel – was kommt eigentlich dabei heraus?: Ein einfacher Maltose-Nachweis am Ende der enzymatischen Hydrolyse von Amylose und die überraschende Anwesenheit von Glucose (in German), MNU Journal 2016, 69, 325–328.




I appreciate this nice clear review of an interesting color test!