High-performance liquid chromatography (HPLC) is an analytical technique to separate, identify, and quantify components in a mixture. It is the main chromatography technique used in most laboratories worldwide.
How Does HPLC Work?
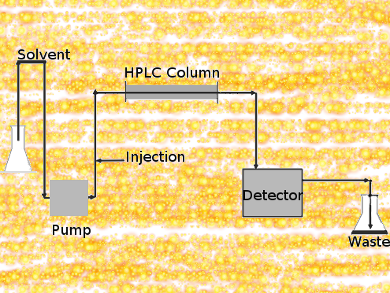
In column chromatography, a solvent drips through a column filled with an adsorbent under gravity. HPLC is a highly improved form of column chromatography. A pump forces a solvent through a column under high pressures of up to 400 atmospheres. The column packing material or adsorbent or stationary phase is typically a granular material of solid particles such as silica or polymers.
The pressure makes the technique much faster compared to column chromatography. This allows using much smaller particles for the column packing material. The smaller particles have a much greater surface area for interactions between the stationary phase and the molecules flowing past it. This results in a much better separation of the components of the mixture.
The pressurized liquid is typically a mixture of solvents such as water, acetonitrile and/or methanol and is referred to as the mobile phase.
The components of a mixture are separated from each other due to their different degrees of interaction with the absorbent particles. This causes different elution rates for the different components and leads to the separation of the components as they flow out the column. Compared to column chromatography, HPLC is highly automated and extremely sensitive.
Types of HPLC
The two most common variants are normal-phase and reversed-phase HPLC.
Normal-Phase HPLC
The column is filled with tiny silica particles, and a non-polar solvent, for example, hexane. A typical column has an internal diameter of 4.6 mm or smaller and a length of 150 to 250 mm. Non-polar compounds in the mixture will pass more quickly through the column, as polar compounds will stick longer to the polar silica than non-polar compounds will.
Reversed-Phase HPLC
The column size is the same. The column is filled with silica particles which are modified to make them non-polar. This is done by attaching long hydrocarbon chains (8–18 C atoms) to its surface. A polar solvent is used, for example, a mixture of water and an alcohol such as methanol. Polar compounds in the mixture will pass more quickly through the column because a strong attraction occurs between the polar solvent and the polar molecules in the mixture.
Non-polar molecules are slowed down on their way through the column. They form varying degrees of attraction with the hydrocarbon groups principally through van der Waals dispersion forces and hydrophobic interactions. They are also less soluble in the aqueous mobile phase components facilitating their interactions with the hydrocarbon groups.
Reversed phase HPLC is the most commonly used form of HPLC.
Also of Interest
- Tips and Tricks for the Lab: Column Packing,
Sarah Millar,
ChemistryViews.org 2012.
The quick and efficient setting up of a column can take years to master. Here are some tips and tricks to set up the perfect column
- More on HPLC
with more personal stories and a wealth of free content




thank you very much for such a simplified explanation of HPLC. I found this interesting. may you continue providing more of these
this was very helpful. thanks
wow.such a great introduction to the HPLC subject.thank you!
This gave a clear and basic idea about the HPLC.. THANK YOU