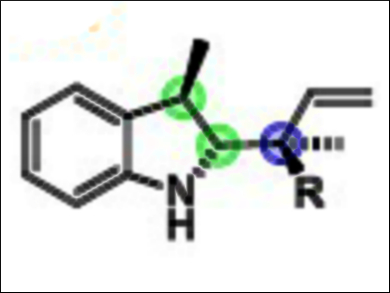
Kálmán J. Szabó, Stockholm University, Sweden, and colleagues have developed a rapid synthesis of chiral nitrogen-containing terpenoids bearing up to three adjacent stereocenters (example pictured). The reaction is a variant of the allylboration reaction, introduced by the late Nobel Laureate H. C. Brown. The approach uses allylboronic acids, derived from the corresponding and naturally occurring terpenoid allylic alcohols.
The developed allylborylation of indoles and dihydroisoquinolines proceeds in one step from achiral starting materials and is stereodivergent. The desired stereoisomer can be produced by choice of the starting materials and the 1,1′-bi-2-naphthol (BINOL) catalyst employed. The use of γ-disubstituted allylboronic acids provides products bearing a quaternary stereocenter.
With this approach, asymmetric catalysis can be used to produce densely functionalized homoallylic amines in high yields in a stereodivergent manner. According to the researchers, these are important features for drug development, where accessing all stereoisomers of a chiral “lead” compound is necessary for evaluating their pharmacological properties.
- Catalytic Asymmetric Allylboration of Indoles and Dihydroisoquinolines with Allylboronic Acids: Stereodivergent Synthesis of up to Three Contiguous Stereocenters,
Rauful Alam, Colin Diner, Sybrand Jonker, Lars Eriksson, Kálmán J. Szabó,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201608605