Paint Mixtures 200 Years Ago
The fluid and loose brushwork used by J. W. M. Turner and other innovative 19th century artists to capture the momentary effects of light was technically made possible by the addition of “gumtion” or “megilp” to the paint matrix, which gave the paints the jelly-like consistency needed for their impasto-rich paintwork. In the journal Angewandte Chemie, scientists unveil the crucial role lead acetate played in this gelation process.
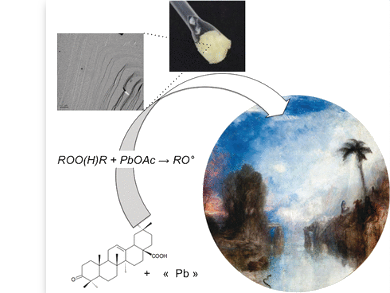
Historic oil paints were based on pigments mixed with oil and resin. Because of this oily consistency, the artists had to deal with very long drying periods for each color layer and production times of months to even years. That J. M. W. Turner could finish his trendsetting painting “The Dawn of Christianity” (pictured above) from 1841 within days was mainly due to a new and innovative paint matrix: “gumtion”, made by mixing of lead acetate, linseed oil, and mastic resin. Its viscoelastic gel properties allowed for the quick subsequent addition of paint layers for the first time—this great facilitation was embraced by the pioneers of modern styles in the 19th century. But what exactly caused gel formation, and how can its chemistry be described today? Laurence de Viguerie from Université Pierre et Marie Curie, Paris, France, and CNRS and her colleagues at the Collège de France, among others have unveiled some of the chemical secrets of gumtion.
What Exactly Caused Gel Formation
Following the original recipes, the researchers found gel formation when mixing lead acetate, linseed or nut oil, and mastic resin dissolved in turpentine. “Today such syntheses can be described as processes that form organic–inorganic hybrid materials which are known for their wide range of applications”, the authors explained. In combination with ancient lake pigments such as madder lake oil paint, the formed gels attain strong elastic properties enabling the artists to paint fast and with thick impasto.
But what exactly causes the gel formation in this mixture of natural materials? Using spectroscopic techniques, the team identified an oxidative free-radical mechanism, similar to the one responsible for the drying and ageing processes in resins and oils. The network-forming processes are just fastened in the presence of a transition metal—which is lead in this case. The results also indicate that the lead not only catalyzes network formation, but it also can take part in the gel architecture, which is a typical inorganic–organic hybrid metallogel.
Thus, it was nothing less than applied chemistry that brought about some of the technical innovations indespensable for making stylistic progress in 19th century paintings.
- A 19th Century “Ideal” Oil Paint Medium: A Complex Hybrid Organic–Inorganic Gel,
Laurence de Viguerie, Maguy Jaber, Hélène Pasco, Jacques Lalevée, Fabrice Morlet-Savary, Guylaine Ducouret, Baptiste Rigaud, Thierry Pouget, Clément Sanchez, Philippe Walter,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201611136