As a molecular chaperone, heat-shock protein 90 (hsp90) is important for maintaining the conformation, stability, activity, and cellular localization of several key oncogenic client proteins, which are involved in signal transduction pathways leading to proliferation, cell-cycle progression, apoptosis, angiogenesis, and metastasis. Hsp90 function may be blocked by specific inhibitors, thereby freezing the chaperone cycle. This decreases the affinity of hsp90 for client proteins, leading to proteasome-mediated degradation of oncogenic client proteins.
Mouâd Alami, Michel Renoir, and co-workers, University of Paris Sud, France, have discovered and synthesized a new compound, 6BrCaQ, which has potent anticancer activity thanks to its ability to destabilize several hsp90 client proteins. This was deduced from a combination of cell viability tests, flow cytometry analyses, and Western blot experiments in a panel of cancer cell lines that allow identification of the apoptotic and autophagic potential of 6BrCaQ. Comparison with one other lead compound (4TCNA), identified previously by Alami’s group, suggests that compounds from this family of hsp90 inhibitors may target a different cluster of hsp90 client proteins.
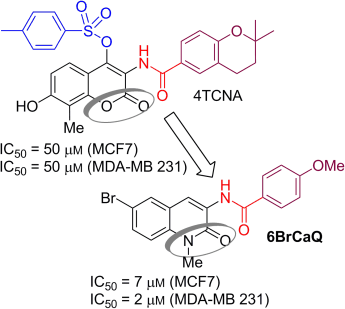
Image: (c) Wiley-VCH
- Discovery and Biological Activity of 6BrCaQ as an Inhibitor of the Hsp90 Protein Folding Machinery
D. Audisio, S. Messaoudi, L. Cegielkowski, J.-F. Peyrat, J.-D. Brion, D. Methy-Gonnot, C. Radanyi, J.-M. Renoir, M. Alami, ChemMedChem 2011, 6(05).
DOI: 10.1002/cmdc.201000489