Conventional hydrogen fuel cells rely on platinum catalysts in order to efficiently produce power from hydrogen and oxygen. The scarcity and consequent high price of platinum prevent the large-scale deployment of such technologies. In nature, metalloenzymes such as hydrogenases and multicopper oxidases with iron, nickel, and copper active sites readily compete with platinum in terms of intrinsic performances towards both hydrogen oxidation and oxygen reduction.
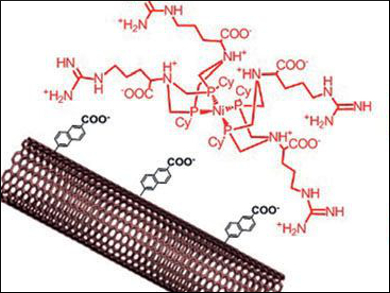
Wendy J. Shaw, Pacific Northwest National Laboratory, Richland, WA, USA, Vincent Artero and Alan Le Goff, University of Grenoble, France, and colleagues have designed novel platinum-free hydrogen fuel cells. The team immobilized biomimetic nickel bis-diphosphine complexes with arginine in the outer coordination sphere (pictured) on modified carbon nanotubes (CNTs) through electrostatic interactions.
The researchers integrated the resulting material either in an enzymatic fuel cell together with a multicopper oxidase at the cathode, or in a proton exchange membrane fuel cell (PEMFC) using Pt/C at the cathode. The cells approach the performance of conventional platinum cells tested under similar conditions and set a new efficiency record for a hydrogen biofuel cell with base metal catalysts.
- Carbon-Nanotube-Supported Bio-Inspired Nickel Catalyst and Its Integration in Hybrid Hydrogen/Air Fuel Cells,
Solène Gentil, Noémie Lalaoui, Arnab Dutta, Yannig Nedellec, Serge Cosnier, Wendy J. Shaw, Vincent Artero, Alan Le Goff,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201611532