Point chirality originates from having four different substituents bonded to a central atom to give nonplanar molecules with nonsuperimposable mirror images. The induction of point chirality in molecular systems is of particular interest in connection with the origin of homochirality in nature, as seen in amino acids.
The interest of Nobuyuki Tamaoki and P. K. Hashim, Hokkaido University, Japan, is to propose a new concept for the introduction of point chirality and to understand how small differences in the substituents on the central carbon atom can cause detectable asymmetry in the structure. They report a new class of azobenzene-based prochiral molecules and the on/off switching of point chirality.
A molecule consisting of a carbon atom having two photoisomerizable azobenzene moieties and a methyl and a benzene group was designed (scheme). The conformational difference caused by the E/Z photoisomerization of one of the azobenzene moieties was successfully utilized for the generation of point chirality in the molecule. The asymmetric center is formed by light-induced changes to the substituents on the central atom, and Z/E thermal isomerization regenerates the initial symmetry in the molecule. The switching of the asymmetry can be performed repeatedly.
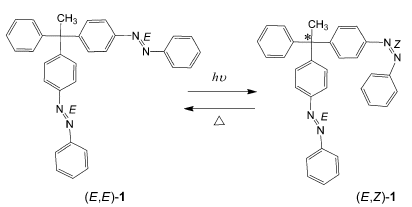
This is the first example of the induction of point chirality in which two of the substituents around an sp3 carbon atom are geometric isomers.
- Induction of Central Chirality by E/Z Photoisomerization,
P. K. Hashim, Nobuyuki Tamaoki
Angew. Chem. Int. Ed. 2011, 50.
DOI: 10.1002/anie.201104614