The ligand-assisted morphology control of nanoparticles has been demonstrated with great success in colloidal synthesis. The ligand pair of oleic acid and oleylamine has previously been used to control the morphology of organic-inorganic hybrid perovskite nanocrystals. Such perovskite-based materials have attracted significant attention due to their useful properties for solar energy conversion.
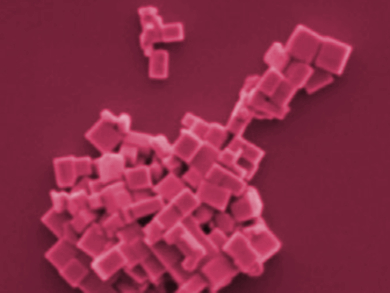
Haifeng Yuan, Maarten B. J. Roeffaers, KU Leuven, Belgium, and colleagues have developed a simple method to tune the nanocrystal morphology of methylammonium lead halide (MAPbX3) and create shapes such as rods, wires, plates, and cuboids (pictured) with sizes of several hundreds of nanometers. The team prepared a lead iodide (PbI2) and methylammonium iodide (MAI) precursor solution in acetonitrile and injected it into a toluene solution containing the capping agents, oleic acid (OAc) and oleylamine (OAm). The morphology of the resulting nanocrystals depends on the ratio of the binary capping agents.
The mechanisms for morphology control are likely associated with the different binding kinetics of the two ligands on different crystal facets. The monocrystalline nature of synthesized nanocrystals was demonstrated using high-resolution scanning transmission electron microscopy (STEM). The employed ligands can stabilize the nanocrystals for several months in the dark. The method could pave the way for the synthesis of high-quality monocrystals with a controllable morphology tuned for different applications.
- Facile Morphology-Controlled Synthesis of Organolead Iodide Perovskite Nanocrystals Using Binary Capping Agents,
Elke Debroye, Haifeng Yuan, Eva Bladt, Wouter Baekelant, Mark Van der Auweraer, Johan Hofkens, Sara Bals, Maarten B. J. Roeffaers,
ChemNanoMat 2017.
DOI: 10.1002/cnma.201700006