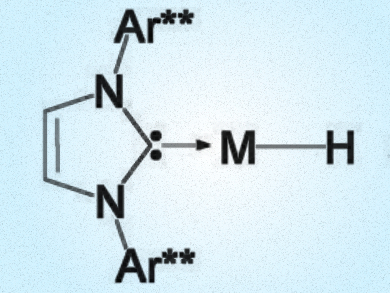
For almost two centuries, copper hydrides have only been known as oligomers (LCuH)n (L = ligand). One prominent representative is the so-called Stryker hexamer [(Ph3P)CuH]6. Monomeric copper hydride complexes have recently been postulated as key intermediates in several different catalytic processes, but had never been spectroscopically characterized.
Guy Bertrand, University of California, San Diego, La Jolla, USA, and colleagues have shown that by using an extremely bulky N-heterocyclic carbene ligand, namely 1,3-bis[2,6-bis[di(4-tert-butylphenyl)-methyl]-4-methylphenyl]imid-azol-2-ylidene (IPr**), it is possible to spectroscopically observe such a monomeric copper hydride. Usinc NMR spectroscopy, the researchers could show that LCuH is in equilibrium with its dimer in solution, whereas only the latter exists in the solid state. This observation supports the hypothesis that copper hydride oligomers dissociate in solution, which rationalizes their high reactivity.
Surprisingly, it was also possible to characterize the corresponding silver hydride monomer both in solution, using NMR spectroscopy, and in the solid state, using X-ray diffraction. Very little is known about silver hydrides, but since they are less prone to oligomerize, the researchers expect their reactivity to be enhanced compared to copper hydrides.
- Spectroscopic Evidence for a Monomeric Copper(I) Hydride and Crystallographic Characterization of a Monomeric Silver(I) Hydride,
Erik A. Romero, Pauline M. Olsen, Rodolphe Jazzar, Michele Soleilhavoup, Milan Gembicky, Guy Bertrand,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201700858