Nematic liquid crystal phases have only one-directional order and are the most disordered liquid-crystal phase, in which rodlike molecules are spinning, sliding, and vibrating. It is thus difficult for the molecules in nematic phases to self-organize.
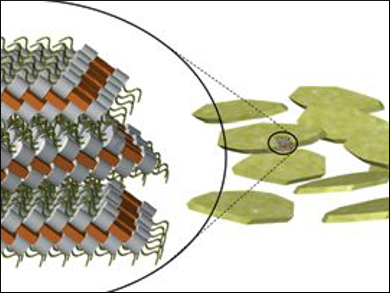
Keiki Kishikawa, Chiba University, Japan, and colleagues have synthesized dumbbell-shaped molecules consisting of only carbon and hydrogen atoms in which two bulky ball-like sites were fixed with a thinner plate (pictured left). The team expected self-organization by intermolecular interlocking of the two sites, even in the nematic phase.
The researchers investigated the phase transitions by a variety of microscopic techniques as well as molecular dynamics simulations. Upon heating to approximately 200 °C, the compound shows an unknown liquid crystal (LC) phase that occurs between two nematic phases (177 °C and 225 °C). The LC phase corresponds to the interlocking of the molecules and has a layered structure. At 225 °C, the layered structure disintegrates to form a nematic phase, comprising particles with a rice-grain shape.
It is surprising that the molecular interlocking process occurs at a high temperature. The technique could become a powerful tool for molecular self-organization even in a highly disordered liquid crystal phases.
- Shape-Assisted Self-Organization in Highly Disordered Liquid Crystal Phases,
Keiki Kishikawa, Yusuke Yamamoto, Go Watanabe, Ayaka Kawamura, Michinari Kohri, Tatsuo Taniguchi,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201700809


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)