Elemental Boron is Effective Photothermocatalyst for Conversion of CO2
A “self-heating” boron catalyst that makes particularly efficient use of sunlight to reduce carbon dioxide serves as a light harvester, photothermal converter, hydrogen generator, and catalyst in one. In the journal Angewandte Chemie, researchers introduce a photothermocatalytic reaction that requires no additives beyond water. This could form the basis of a new, more efficient process for converting the greenhouse gas CO2 into a useful carbon source for the production of fuels and chemical products.
Decades of Research to Make CO2 Useful
The ideal route for making CO2 useful is considered to be reduction aided by a photocatalyst to use sunlight as the only source of energy—a process that corresponds to the first step of photosynthesis. Despite decades of research, processes for converting CO2 are still too inefficient. “This is largely due to the insufficient utilization of solar light, the high energy barrier for CO2 activation, and the sluggish kinetics of the multiple electron and proton transfer processes,“ explains Jinhua Ye.
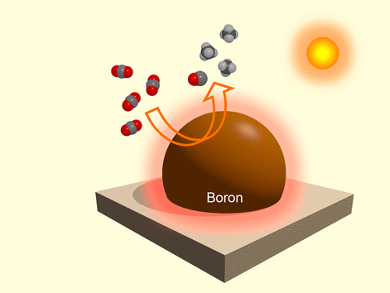
Working with a team for the National Institute for Materials Science (NIMS) in Tsukuba, Ibaraki, and Hokkaido University in Sapporo, Japan, as well as Tianjin University and Nanjing University of Aeronautics and Astronautics, China, Ye is pursuing a strategy that uses both the light and thermal energy provided by sunlight. When the sun shines on a surface, it is heated. The researchers want to use this ordinary photothermic effect to increase the efficiency of catalytic systems. Their material of choice is powdered elemental boron, which very strongly absorbs sunlight and efficiently converts it photothermically, heating itself up remarkably. This allowed the team to carry out the efficient reduction of CO2 to form carbon monoxide and methane under irradiation in the presence of water, with no additional reagents or co-catalysts.
Photothermocatalytic Strategy for the Conversion of CO2
Irradiation causes the boron particles to heat up to about 378 °C. At this temperature it reacts with water, forming hydrogen and boron oxides in situ. The boron oxides act as “traps” for CO2 molecules. The hydrogen is highly reactive and, in the presence of the light-activated boron catalyst, efficiently reduces the CO2 by providing the necessary protons and electrons.
“The key to our success lies in the favorable properties of the boron powder, which make it an all-in-one catalyst: light harvester, photothermic converter, hydrogen source, and catalyst,” says Ye. “Our study confirms the highly promising potential of a photothermocatalytic strategy for the conversion of CO2 and potentially opens new vistas for the development of other solar-energy-driven reaction systems.”
- Elemental Boron for Efficient Carbon Dioxide Reduction under Light Irradiation,
,
Angew. Chem. Int. Ed. 2017.
DOI: https://doi.org/10.1002/anie.201701370