Nicotinoids play an important biochemical and pharmacological role as agonists of acetylcholine receptors. These receptors are found not only in cells of the nervous system but also in skeletal muscles. Their activation leads to cation-selective flux through the receptor, leading to the excitation of neurons or the regulation of intracellular signaling cascades.
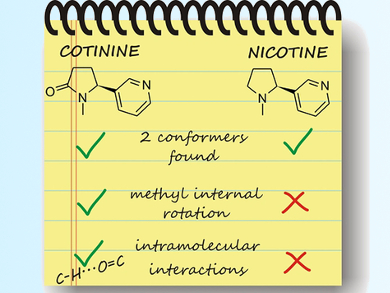
The parent molecule nicotine and a keto-derivative, cotinine, are common nicotinoids being studied. These nicotinoids can be seen as an assembly of pyridine and N-methyl-pyrrolidine (in the case of nicotine) or -pyrrolidinone (in the case of cotinine) rings linked by a single C–C bond (pictured). Pharmacologically, cotinine shows a reduced effect compared to nicotine, however, the structure-activity relationship has not been fully elucidated.
Emilio J. Cocinero, Universidad del Pais Vasco, Bilbao, Spain, and colleagues used a combination of rotational spectroscopy and supersonic jet expansions to characterize the gas-phase conformations of nicotine and cotinine at a high resolution. They used computational methods to confirm the experimental results. The team found two low-energy conformations in which the pyridine ring is an equatorial substituent of the pyrrolidine/-one. Furthermore, the methyl substituent on the pyrrolidine/-one ring can rotate in cotinine, in contrast to its behavior in nicotine. Lastly, the carbonyl substituent in cotinine can also create an intramolecular hydrogen bond.
All of these conformational differences may affect cotinine’s affinity to acetylcholine receptors. Although processes in living cells happen in aqueous media, gas phase studies offer the chance to characterize single molecule structural properties and their processes, while avoiding the complexity of interactions found in real biological systems.
- Structural Studies of Nicotinoids: Cotinine versus Nicotine,
Iciar Uriarte, Cristóbal Pérez, Elena Caballero-Mancebo, Francisco J. Basterretxea, Alberto Lesarri, José A. Fernández, Emilio J. Cocinero,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201700023