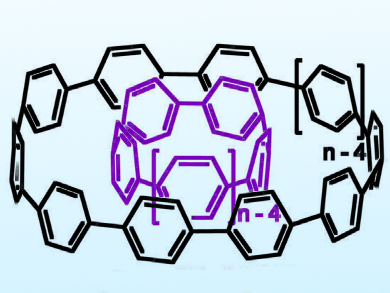
Multiwalled carbon nanotubes (CNTs), which consist of multiple rolled layers (concentric tubes) of graphene, have interesting mechanical, electrical, optical, and thermal properties. Owing to the potential applications of these materials, it is important to better understand CNTs on the molecular level. Cycloparaphenylenes (CPPs) are good model compounds for this purpose because these nanorings are the smallest possible structures corresponding to the sidewalls of CNTs. It had been predicted that nanorings of different sizes would form host–guest complexes stabilized by attractive van der Waals interactions.
Shigeru Yamago, Kyoto University, Japan, and colleagues have found that CPPs form host–guest complexes (example pictured) selectively: when a CPP containing six phenylene units, [6]CPP, is mixed with equimolar amounts of the larger [8]–[12]CPPs, the only complex that forms is that in which [6]CPP is nested inside [11]CPP, denoted as [11]CPP⊃[6]CPP. The team’s work indicates that in other favored combinations, the inner and outer rings differ in size by five phenylene units. The ternary complex [15]CPP⊃[10]CPP⊃C60 displays a “planetary orbit” structure. In all of these complexes, the difference in the diameters of the nesting aromatic hydrocarbons, 0.35 Å, corresponds to the distance separating the curved graphene layers in CNTs.
These models provide support for the proposed interlayer interactions in CNTs. In future work, CPPs could be tailored further to probe the electronic properties in layered architectures such as CNTs.
- Shortest Double-Walled Carbon Nanotubes Composed of Cycloparaphenylenes,
Sigma Hashimoto, Takahiro Iwamoto, Daisuke Kurachi, Eiichi Kayahara, Shigeru Yamago,
ChemPlusChem 2017.
DOI: 10.1002/cplu.201700097This article is part of a special issue of ChemPlusChem on “Novel Aromatics: From Synthesis to Applications”.