Unraveling supramolecular self-assembly processes in solution can be difficult. NMR techniques can be useful, but they often give limited information about how two or more components are interacting, only that they are interacting.
Megan O’Mara and Nicholas White, Australian National University, Canberra, and colleagues have used a combination of molecular dynamics (MD) simulations, NMR spectroscopy, and X-ray crystallographic experiments to probe the interactions between simple bis(amidinium) cations and dicarboxylate anions. They observed a wide range of assemblies in solution, including tape-like arrangements and several different cyclic structures, and rapid exchange between the different dynamic structures. In part this is due to a range of hydrogen bonding interactions which are possible between the amidinium and carboxylate moieties
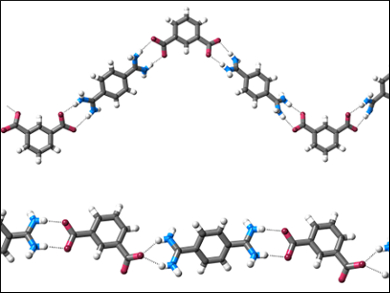
Crystallization reduced the dynamic equilibria to a single solid state product. Mainly 1D chain structures (pictured) form; they are held together by short N−H⋅⋅⋅O hydrogen bonds. Because of the good agreement between computational and experimental techniques, the team suggests that their approach could be used to gain insight into more complex supramolecular self-assembly processes.
- Hydrogen bond-Driven Self-Assembly between Amidinium Cations and Carboxylate Anions: A Combined Molecular Dynamics, NMR Spectroscopy, and Single Crystal X-ray Diffraction Study,
Michael Thomas, Thomas Anglim Lagones, Martyna Judd, Mahbod Morshedi, Megan L. O’Mara, Nicholas G. White,
Chem. Asian J. 2017.
DOI: 10.1002/asia.201700406