Black phosphorus is a promising semiconductor with possible applications in nanoscale electronic devices. The 2D material has a bandgap, unlike graphene, and a higher electron mobility than silicon. These properties could be tuned by chemically modifying black phosphorus.
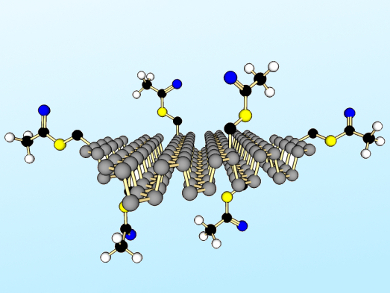
Zdeněk Sofer, Institute of Chemical Technology Prague, Czech Republic, Martin Pumera, Nanyang Technological University, Singapore, and colleagues have developed a method to chemically modify layered black phosphorus. Refluxing suspensions of black phosphorus (pre-exfoliated by shear force milling) were treated with nucleophiles, e.g., S-(bromomethyl) ethanethioate, to form covalent P–C and P–O–C bonds (example product pictured). Other functionalization routes were less effective, including those involving radical species, Grignard reagents, and organolithium compounds.
Using ab initio calculations, the team verified that covalent P–C and P–O–C modification has a strong effect on the electronic structure of layered black phosphorus and single-layer phosphorene. These results suggest the 2D material can be tailored for diverse applications in electronics and optoelectronics.
- The Covalent Functionalization of Layered Black Phosphorus by Nucleophilic Reagents,
Zdeněk Sofer, Jan Luxa, Daniel Bouša, David Sedmidubský, Petr Lazar, Tomáš Hartman, Hilde Hardtdegen, Martin Pumera,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201705722