Boron neutron capture therapy (BNCT) is an emerging technique against cancer. It relies on the release of high-energy particles from non-radioactive compounds containing boron-10 upon irradiation with epithermal neutrons (i.e., those with an energy of 0.025–0.4 eV). Because the trajectory of these particles is in the magnitude of a cell’s diameter, the BNCT damage is confined within the tumor, as long as the BNCT agents are specifically localized in tumor tissues.
Hugo Cerecetto, Universidad de la República, Montevideo, Uruguay, Clara Viñas, Institut de Ciències dels Materials de Barcelona, Bellaterra, Spain, and colleagues have hybridized boron-rich carboranyl clusters with erlotinib, a tyrosine kinase receptor (TKR) inhibitor to form a series of possible anticancer compounds, varying in the linkage position and linker molecules (example pictured below).
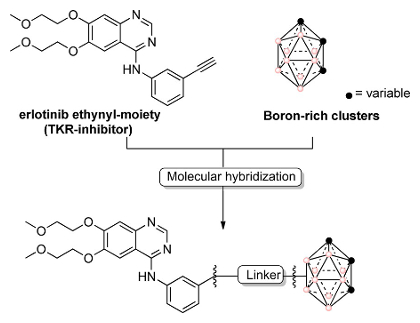
The team then tested the hybrids against C6 glioma cells (brain tumor cells), and found that the hybrids exhibit anticancer activity that is dependent on their lipophilicity. Erlotinib alone did not show significant anticancer activity. It has an IC50 value (a measure of the compound’s effectiveness) of over 100, compared with 30 μM and 34 μM, respectively, for the two most active hybridized compounds.
The researchers also tested the most active compounds against primary glial cells (healthy brain cells) and showed that the compounds are selective towards cancer cells. This makes them promising anticancer agents with dual action: as TKR inhibitors (based on the erlotinib unit) and as BNCT agents (based on the boron-rich clusters which can capture neutrons).
- Small-Molecule Kinase-Inhibitors-Loaded Boron Cluster as Hybrid Agents for Glioma-Cell-Targeting Therapy,
Marcos Couto, Ignacio Mastandrea, Mauricio Cabrera, Pablo Cabral, Francesc Teixidor, Hugo Cerecetto, Clara Viñas,
Chem. Eur. J. 2017, 23, 9233–9238.
DOI: 10.1002/chem.201701965




