Earth-abundant transition metals are preferred to noble metals because they are environmentally benign and have a lower cost. Nickel catalysts, for example, are attractive for organic synthesis
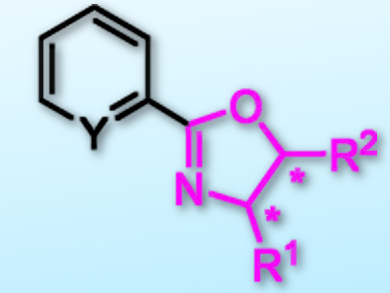
Inspired by nickel-catalyzed C–H bond functionalizations of oxazoles, Zhan Lu, Zhejiang University, Hangzhou, China, and colleagues have found that NiBr2 can efficiently catalyze C–H arylations of oxazolines. The team used NiBr2 together with 1,2-bis(diphenylphosphino)ethane (dppe) as a ligand in the presence of tBuOLi in toluene at 120 °C. Various aryl or heteroaryl halides (-Cl, -Br, -I) and oxazolines could be used as partners for the synthesis of chiral oxazoline-containing multidentate ligands (example pictured).
This method is particularly useful for the construction of the chiral oxazoline iminopyridine (OIP) ligand, which has been widely used in asymmetric hydrofunctionalizations of unsaturated C–C bonds or C–O double bonds. Moreover, a frequently used pybox ligand could be synthesized smoothly under these reaction conditions.
- Nickel-Catalyzed C−H Heteroarylation of Chiral Oxazolines,
Peng Lu, Chong-Lei Ji, Zhan Lu,
Asian J. Org. Chem. 2017.
DOI: 10.1002/ajoc.201700446