In public and private buildings as well as in transport vehicles, people are inevitably confronted with a broad spectrum of foreign substances, via both the air and dust residues. This time, we take a look at air exchange and at nanoparticles released by household appliances.
3. Indoor Climate and Comfort
Air-hygienic relationships in a closed room differ from those in the immediate surroundings, constituting unique, delimited atmospheres that one might also characterize as “microclimates”. The structural and artificially ventilated nature of an interior space is crucially influenced and shaped by the regional climate [25].
The indoor climate, in turn, influences the organism of someone within it and can have both positive and negative effects from a health standpoint. In particular, thermal factors and the material composition of the associated air will affect a person’s physiology, and therefore determine to some extent his or her well-being.
With respect to indoor climate, most people display little flexibility. Even small fluctuations in temperature and air movement can trigger discomfort. Moreover, heat sensitivity is highly dependent on such things as bodily activity, clothing, air temperature, mean radiant temperature, air velocity, and humidity. But even adherence to all standard comfort criteria cannot automatically guarantee an occupant’s sense of well-being. Although humidity directly influences one’s overall sense of well-being, it is also of hygienic significance: high humidity contributes to the growth of microorganisms, and thus enhances microbiological processes.
3.1. Air Exchange in an Interior Space
From both structural and hygienic points of view, a consistent exchange of air in interior spaces is essential. Poor ventilation has particularly drastic effects in rooms with a high occupancy rate. It is often necessary that numerous air exchanges occur every hour in order to adequately remove carbon dioxide generated by exhalation.
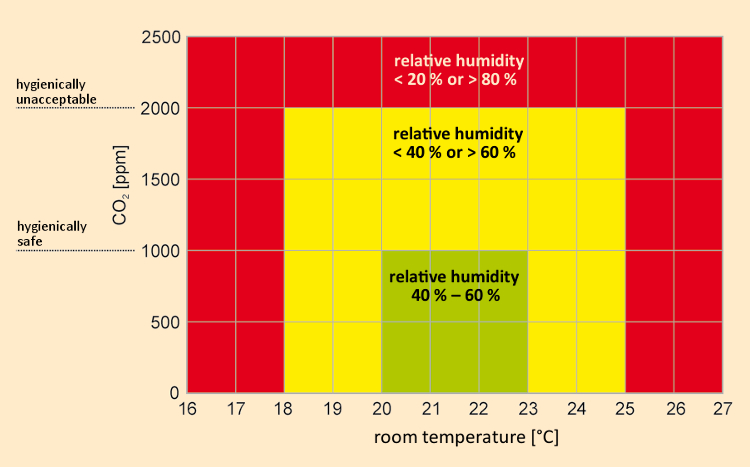
Considering hygienic aspects, the CO2 concentration in room air constitutes an important indicative parameter for air contamination due to human activity. The policy statement of the Commission for Interior Space Benchmarks (abbreviated as “AIR” in German) considers a CO2 concentration lower than 1000 ppm to be hygienically harmless, but a value above 2000 ppm hygienically unacceptable. Figure 5 shows thermal domains regarded as comfortable (green), acceptable (yellow), and unacceptable (red) based on examples drawn from classrooms [26].
 |
|
Figure 5. Definitions of various thermal comfort zones, using the example of a classroom [26]; the green region is regarded as optimal and the yellow region as acceptable from a hygienic perspective, but the red region is hygienically unacceptable. The CO2 criteria correspond to the Commission for Interior Space Benchmarks [5]. |
In order to discuss intelligently the concept of air exchange within an internal space, it is necessary to agree upon an interpretation for the air exchange number n. According to equation (3), this is defined as the quotient of inlet-air volume flow rate (m3/h) divided by room volume:
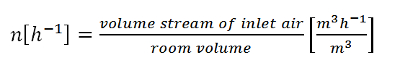 |
(3) |
3.2. Air Purifiers
An air purifier––that is, a device with which air quality in an enclosed space is supposed to be improved after the fact––needs to be viewed critically from a chemical point of view. The decomposition of substances considered to be air contaminants is typically initiated either with ozone or by a photocatalytic process. In particular, it is mainly odiferous, i.e., smelly, substances that one wishes to remove.
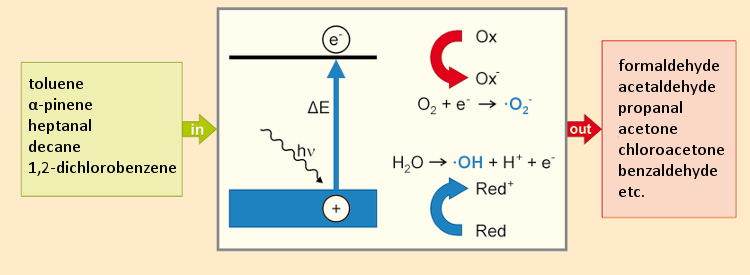
The principle of photocatalytic air purification is often based on the use of nanoscale amounts of titanium dioxide as a semiconductor. The energy difference in the band gap for excitation of an electron from the TiO2 valence band to the conduction band is 3.2 eV (387 nm); see Figure 6. An electron in the conduction band reduces oxygen to the superoxide anion (·O2–). Decomposition of water allows the valence band once again to acquire its missing electron, with formation of a hydroxyl radical (·OH). The reactive oxygen species (ROS) then reacts with organic components in the air. This normally does not lead to complete mineralization of the starting materials (to products like CO2 and water), so some oxidized secondary products are released.
 |
|
Figure 6. Operating principles of a photocatalytic air-purification device. |
Through test-chamber studies with various devices and five different starting materials (highlighted in green in Fig. 6), a large number of secondary products were detected (highlighted in red), some of them in high concentrations [27]. For this reason, the Internal Space Hygiene Commission of the German Federal Environmental Agency currently advises against the use of such air-purifying devices.
There are also wall paints on the market which, through nanoscale doping with titanium dioxide, allegedly offer an effective photocatalytic effect under both normal daylight and artificial lighting conditions. But if the paint itself contains organic components (e.g., binding agents based on acrylates), then these tend to react first, with a release of volatile components into the room air. The air-purifying principle is thereby reduced to absurdity [28].
4. How Dangerous Is My Household Appliance?
The burden on room air from ultrafine particles has been a subject of concern for years, as adequately documented through countless studies. In what follows, we present a few examples of nanoparticle release due to household appliances.
4.1. Toasters
In the context of a measuring program undertaken by the Fraunhofer Institute for Wood Research (Wilhelm-Klauditz-Institut, WKI), Braunschweig, Germany, typical household devices like toasters, grills, microwaves, electric irons, and hair dryers were studied inside test chambers [29]. Both cheap and expensive items were studied and compared. The tested devices were all new, so as to exclude potential problems arising from contamination due to previous use, such as food residues or oils.
 |
|
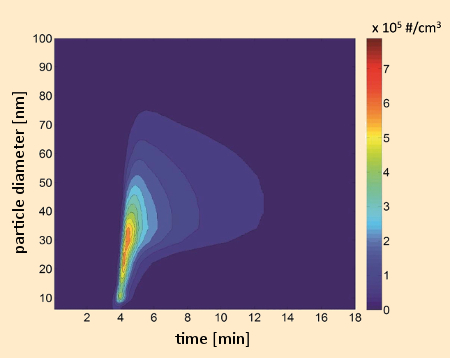
Figure 7. Time-dependent course of the release of nanoparticles during two-minute operation of a toaster (without bread) in a 1 m3 emission chamber at 23 °C, 50 % relative humidity, and an air exchange rate of 3 h–1. The particle-size distribution measurement was accomplished with a fast-mobility particle analyzer over the range 5.6–560 nm. An increase in particle size through agglomeration is easily recognizable [29]. |
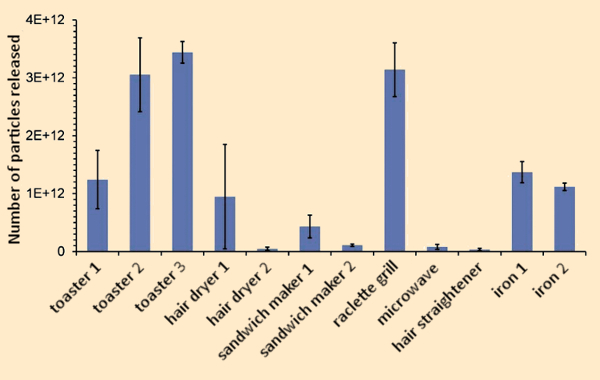
From Figure 7 it is evident that with a toaster, for example, most of the relevant particles produced have diameters smaller than 50 nm. Upon release into the test-chamber air, these particles grow via agglomeration. If one then compares the numbers of particles released per use, significant differences are apparent between various devices (see Fig. 8). At temperatures between 150 °C and 200 °C, these particles turned out to be vaporizable. This suggests that they are organic in nature, and formed by condensation of chemical substances released by components of the appliances.
 |
|
Figure 8. Particle release from various household devices during typical operating periods in a 1 m3 emission chamber under identical conditions (see Fig. 7). Reported is the sum of particles detected in the size range 5.6–560 nm. The error bars represent standard deviations from three measurements in each case. |
4.2. Restaurants
Wallace and Ott [30] came upon the truly intelligent idea of studying particle appearance in the course of various gourmet events. It turned out, for example, that the frying of shrimp led to similar particle concentrations as preparing scrambled eggs and ham.
One idea that clearly warranted a creativity prize was feasting at a total of 22 different restaurants on the US West Coast (in California) and concurrently making particle measurements. What sounded at first rather quirky, in fact had a realistic and serious background: restaurant guests and amateur cooks certainly need not fear for their health, but at the same time, for restaurant employees, a constant exposure to particles could hardly be beneficial to the respiratory tract, as Buonanno et al. were able to verify in the case of pizzerias [31].
4.3. Offices
A dispute that could almost be described as bizarre has raged for some time regarding the release of nanoparticles from office equipment, especially laser printers. It is undisputed that laser printers do release, during normal operation, nanoparticles into the air. The question is simply: what do these particles consist of, and would exposure to them be expected to present any threat to human health?
Critics insist stubbornly that the particles emitted consist of toner components. Since toners often contain iron oxides, there have occasionally been bizarre allusions to “iron harpoons”, which upon inhalation could bore their way through alveoles and cell walls. That iron oxides are also common components of desert sand is, of course, freely overlooked.
Various studies have shown that with a properly functioning laser printer, the particles which are released are vaporizable, and thus volatile. Based on analyses, the particles contain mainly siloxanes from lubricants related to printer operation, and chemical components of paper, as well as C21 to C45 paraffins present in toner cartridges, where they function as hydrophobicity agents [32]. The fraction of material representing metals or toner particles amounts to only 1–2 % [33]. Naturally, in copy centers, toner components can indeed be found in the air and dust [34], but this will mainly be due to abrasion from bad copies frantically pulled from a printer following a paper jam.
4.4. Incandescent Bulbs and Compact Fluorescent Lamps
The fate of the classic incandescent light bulb, which has been with us for more than a century, was already sealed at the time of its original market launch. In 1900, Max Planck formulated the radiation law bearing his name that shows a tungsten filament glowing at 2500 K to be a power guzzler, emitting most of its radiation in the infrared region.
Thanks to a ban on such light bulbs, applicable throughout the European Union (EU), the market share of energy-saving bulbs, especially compact fluorescent lamps (see Fig. 9), has recently grown dramatically. It is indisputable that this type of lamp operates with considerably greater energy efficiency than an incandescent bulb.
 |
|
Figure 9. Compact fluorescent lamp. |
What the EU has rarely taken into account, however, is that each compact fluorescent lamp contains 1–2 mg of mercury. Their operating principle is as follows: electrons released from a cathode cause mercury atoms to emit UV radiation. This is subsequently transformed by a fluorescent pigment (with which the glass walls of the lamp are coated) into visible light. But if such a lamp should break, the air in the room almost immediately becomes contaminated with mercury.
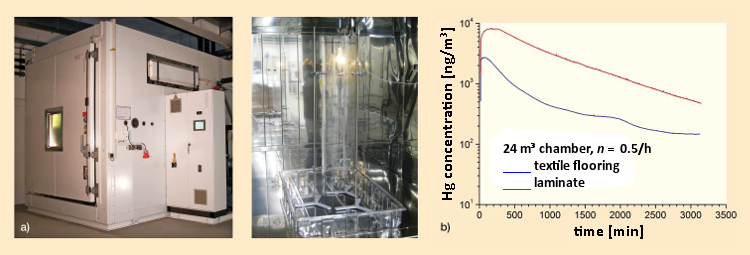
An experimental setup in a 24 m3 stainless steel chamber for studying concentration vs. time behavior is shown in Figure 10a. A mechanical impulse induced from outside causes a lamp to be destroyed. The fragments fall into a plastic basin equipped with a flooring material of either laminate or some sort of textile. A plexiglass cylinder prevents glass fragments from flying around the chamber. Concentration measurements occur online. Gas-phase mercury in the air is first amalgamated with gold, and after subsequent thermodesorption in an optical cell, is analyzed at 254 nm by atomic absorption spectrometry.
 |
|
Figure 10. a) A compact fluorescent lamp (11 W, 1.5 mg Hg) has been turned on and placed in a 24 m3 stainless steel chamber (external view left, internal view right) at 23 °C with a relative humidity of 50 %, and an air exchange rate of 0.5 h–1. |
As shown in Figure 10b, subsequent to shattering of the compact fluorescent bulb, the mercury concentration in the chamber air is a function not only of the mercury content of the lamp, but also of any background level. If the fragments fall on laminate (red curve), there is a high initial concentration of ca. 8000 ng/m3, which decays exponentially. In the case of a textile lining (blue curve), the initial concentration is lower, but the decay behavior becomes more gradual. Textile flooring-surfaces function as reversible sinks, in which the mercury is first absorbed and then slowly released again [35].
In Germany, there are stringent room-air mercury benchmarks, which greatly simplifies the assessment process. Above an air concentration of 0.35 μg/m3, it is recommended that the space is no longer used [36]. After potential mercury problems have become known, the Federal Environmental Office has ordered that extensive investigations be conducted. Appropriate guidance has been prepared in the case of a shattered lamp within a household.
It remains to be noted in a positive sense that the compact fluorescent lamp represents only a temporary or transitional solution. It is to be anticipated (and hoped!) that in the short or long run the clearly more environmentally friendly LED bulb will become firmly established.
References
[25] T. Salthammer, Critical evaluation of approaches in setting indoor air quality guidelines and reference values, Chemosphere 2011, 82, 1507–1517. https://doi.org/10.1016/j.chemosphere.2010.11.023
[26] T. Salthammer et al., Children’s well-being at schools: Impact of climatic conditions and air pollution, Environ. Int. 2016, 94, 196–210. https://doi.org/10.1016/j.envint.2016.05.009
[27] J. Gunschera et al., Portable photocatalytic air cleaners: efficiencies and by-product generation, Environ. Sci. Pollut. Res. 2016, 23, 7482–7493. https://doi.org/10.1007/s11356-015-5992-3
[28] T. Salthammer et al., Photocatalytic Surface Reactions on Indoor Wall Paint, Environ. Sci. Technol. 2007, 41, 6573–6578. https://doi.org/10.1021/es070057m
[29] T. Schripp et al., Characterization of particle emission from household electrical appliances, Sci. Total Environ. 2011, 409, 2534–2540. https://doi.org/10.1016/j.scitotenv.2011.03.033
[30] L. Wallace et al., Personal exposure to ultrafine particles, J. Expos. Sci. Environ. Epidemiol. 2011, 21, 20–30. https://doi.org/10.1038/jes.2009.59
[31] G. Buonanno et al., Exposure to particle number, surface area and PM concentrations in pizzerias, Atmos. Environ. 2010, 44, 3963–3969. https://doi.org/10.1016/j.atmosenv.2010.07.002
[32] T. Salthammer et al., Aerosols generated by hardcopy devices and other electrical appliances, Environ. Pollut. 2012, 169, 167–174. https://doi.org/10.1016/j.envpol.2012.01.028
[33] M. Barthel et al., XRF-Analysis of Fine and Ultrafine Particles Emitted from Laser Printing Devices, Environ. Sci. Technol. 2011, 45, 7819–7825. https://doi.org/10.1021/es201590q
[34] D. Bello et al., Physicochemical and morphological characterisation of nanoparticles from photocopiers: implications for environmental health, Nanotoxicology 2013, 7, 989–1003. https://doi.org/10.3109/17435390.2012.689883
[35] T. Salthammer et al., Estimating human indoor exposure to elemental mercury from broken compact fluorescent lamps (CFLs), Indoor Air 2012, 22, 289–298. https://doi.org/10.1111/j.1600-0668.2011.00764.x
[36] B. Link, Richtwerte für die Innenraumluft – Quecksilber (in German), Bundesgesundheitsblatt 1999, 42, 168–174. https://doi.org/10.1007/s001030050080
The article has been published in German as:
- The Air that I Breathe – Der Innenraum als chemischer Reaktor,
Tunga Salthammer,
Chem. unserer Zeit 2017.
https://doi.org/10.1002/ciuz.201700779
and was translated by W. E. Russey.
The Air that I Breathe – Part 1
Chemistry of indoor air is a relatively new, diverse and interdisciplinary field surrounding us every day
The Air that I Breathe – Part 2
How dangerous are my household appliances?
The Air that I Breathe – Part 3
Emissions of e-cigarettes and other drugs
The Air that I Breathe – Part 4
Man as a source of substances, life on Mars, and what to learn from movies
The Air that I Breathe – Part 5
How does climate change affect indoor air quality?
See similar articles by Klaus Roth published in ChemViews Magazine




