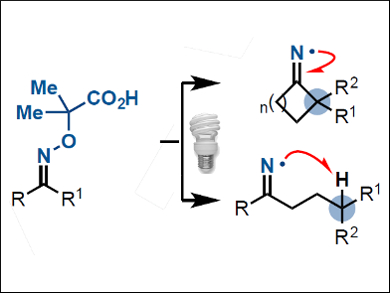
The selective functionalization of non-activated carbon atoms in organic molecules is challenging—especially where densely functionalized molecules are concerned. New strategies are required to target carbon atoms that cannot be accessed by classical methods.
Daniele Leonori, University of Manchester, UK, and colleagues have designed a class of oximes (pictured) that are activated by light-emitting diodes (LEDs) in the presence of an organic photocatalyst. Activation of the oximes with blue light triggers a cascade process that generates highly reactive iminyl radicals. Radical transposition by C–C and C–H bond cleavages gives access to carbon radicals that can undergo further fluorination, chlorination, or azidation to form a wide range of nitrile- and ketone-containing products.
The team used the method to selectively deconstruct and functionalize the complex molecule terpene isosteviol and a precursor of drospirenone, which is an active ingredient in birth control pills. The researchers believe that the method could streamline the exploration of bioactive substrates by simplifying the preparation of molecular libraries used for biological screening.
- Photoinduced Remote Functionalizations via Iminyl Radical-Promoted C–C and C–H Bond Cleavage Cascades,
Elizabeth M. Dauncey, Sara P. Marcillo, James J. Douglas, Nadeem S. Sheikh, Daniele Leonori,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201710790