In public and private buildings as well as in transport vehicles, people are inevitably confronted with a broad spectrum of foreign substances, via both the air and dust residues. In this part, we take a look at emissions from e-cigarettes and drugs.
5. Emissions from E-Cigarettes
E-cigarettes enjoy increasing popularity these days as “lifestyle products”. Consumers of e-cigarettes tend to refer to themselves not as “smokers”, but rather as “vapers”. The vaping lobby is tireless in extolling the advantages of e-cigarettes over the classic tobacco variety, though to some extent on the basis of questionable arguments.
In the film “The Tourist”, Johnny Depp is seen to consume an e-cigarette in the course of a train journey to Venice, causing his companion Angelina Jolie to point out to him that smoking is forbidden in the train. He responds that there is no problem, however, since he is exhaling only steam, a pronouncement that, while convenient, is unfortunately not strictly true.
 |
|
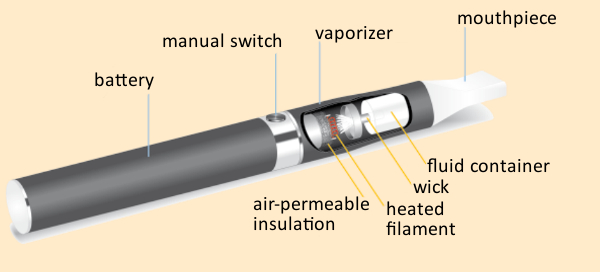
Figure 11. Operating principle of an e-cigarette. |
The way an e-cigarette works is conceptually quite simple (see Fig. 11). A liquid that is to be vaporized passes into a wick via capillary action. Here, vaporization takes place with the aid of a battery-driven heated filament. The resulting vapor can then easily be inhaled. Activation of the filament is achieved either manually by pushing a button, or with a pressure-sensor adjusted to react to the user’s suction. The liquid itself typically consists of a mixture of the two carrier substances propylene glycol and glycerin, in which flavoring agents and nicotine have been dissolved.
5.1 Substance Release
With respect to room-air quality, the question arises whether undesirable substances are released into the surrounding atmosphere through the consumption of e-cigarettes, which is to say whether—analogous to second-hand smoke—there also exists an undesirable “second-hand vaping” phenomenon.
In the case of electronic cigarettes, substances are released into room air only by exhalations from the “vaper”. Sources analogous to the side-stream smoke from a smoldering tobacco cigarette are in this case truly negligible, since liquid from an e-cigarette is vaporized only when the consumer activates the device. The vapor cloud visible during exhalation is due only to propylene glycol, through the formation of fine and ultrafine aerosols [37].
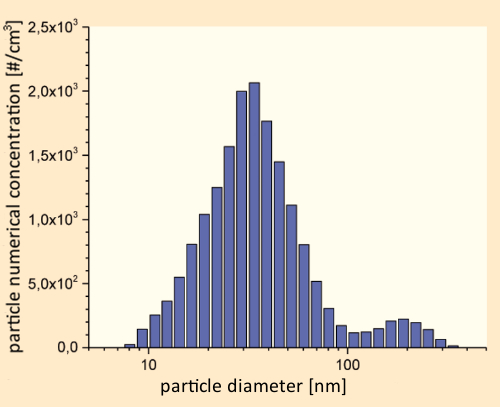
A female subject seated in a test chamber was directed to “vape” an e-cigarette charged with various liquid mixtures. Measurements on the resulting particle-size distributions were carried out with the aid of a fast-mobility particle analyzer. Irrespective of the flavoring agent(s) present, and of the nicotine content, the general size-distribution pattern illustrated in Figure 12 was consistently observed [37].
 |
|
Figure 12. Size distribution in an e-cigarette aerosol within an 8 m3 emission chamber at 23 °C, 50 % relative humidity, and an air exchange rate of 0.3 h–1. |
Propylene glycol vapor is quite frequently employed at concerts and theatrical presentations as a way of simulating fog effects. It has been shown, however, that employees in the entertainment industry who are exposed to such propylene glycol-containing aerosols on a regular basis are more often subject to respiratory tract irritation than people not so exposed [38]. Moreover, the use of e-cigarettes in a room for several hours results in propylene-glycol air concentrations significantly exceeding the 0.07 mg/m3 limit proposed preliminarily by the Commission for Interior Space, a value that for precautionary reasons should probably be adhered to [39]. Furthermore, e-cigarette vapors from most of the vaping liquids in question do contain at least traces of a broad spectrum of organic substances. To some extent, these substances correlate directly with components of the liquids themselves, but some originate in the course of the vaporization process. Nicotine is also clearly present in room air where e-cigarettes have been “vaped”.
5.2 Comparison to Tobacco Cigarettes
In general, emissions from e-cigarettes are indeed lower than those from classical tobacco cigarettes; nevertheless, e-cigarettes definitely cannot be regarded as emission-free, and they clearly contribute to pollution of room air in the form of gaseous organic compounds and aerosols. The Interior Space Hygiene Commission of the German Federal Environmental Office regards such vapors as a threat to the health of third parties, and for this reason recommends that regulations and limitations already applicable to conventional tobacco smoke be maintained with respect to e-cigarette emissions, urging their incorporation into the “non-smoker protection laws” of the German federal government and states [40].
6. Who Lived in My Home Before Me? — Crystal Meth in Residences
Especially in the United States, the number of active small meth-labs has increased dramatically in recent years. So-called “shake and bake” synthetic methods are the ones most often employed, wherein ephedrine is transformed into N-methylamphetamine (commonly called “methamphetamine” or simply “crystal meth”) with the aid of lithium and ammonia in organic solvents [41]. The product itself is precipitated and ultimately consumed in the form of its hydrochloride. The reaction process not only leads to various byproducts [42], but the reaction mixture itself can be extremely dangerous and sometimes explode upon contact with water or air.
Out of fear of being discovered, operators of these small meth labs tend to move frequently. Poppendieck et al. estimate, based on data from the Drug Enforcement Agency (DEA), that in 2010–2012 in the United States alone ca. 40,000 accidents occurred in illegal meth labs [43]. It follows that a great many residences have become contaminated with methamphetamine, its byproducts, and other associated chemicals.
Both methamphetamine and its hydrochloride are nonvolatile. The boiling point of methamphetamine is around 215 °C and the vapor pressure of the supercooled melt is 19 Pa at 298 K. Therefore, methamphetamine is unlikely to be removed simply through ventilation, instead becoming widely distributed over various surfaces.
6.1 Measurements
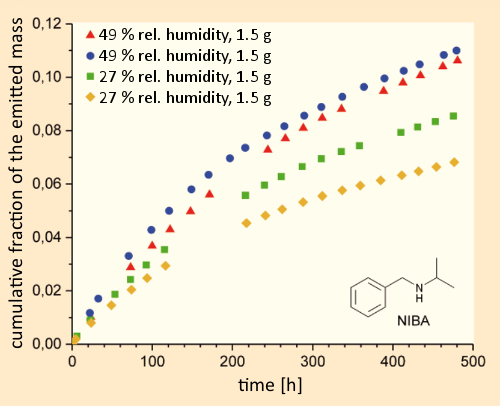
In experiments with the substitute material N-isopropylbenzylamine (NIBA) [43] (which does not fall under drug-control regulations), and a test chamber whose walls consisted of painted plasterboard, ca. three weeks (480 hours) after dosing with the test substance under simulated interior-room conditions (air exchange 3.5 h–1, 32 °C, 49 % humidity, room volume 16.5 L), at most 11 % of the NIBA had been removed via air exchange. In a comparable experiment at a lower humidity level (27 %), the removal was only 7–8 %; i.e., significantly less (see Fig. 13).
 |
|
Figure 13. Crystal meth in a residence. |
The molecular weight of NIBA is identical to that of methamphetamine (149.2 g/mol), while its boiling point of 200 °C is somewhat lower. NIBA is therefore extremely well-suited as an experimental substitute for methamphetamine when considering dynamic behavior in an interior space.
In further experiments, attempts were made to “fix” the NIBA by applying a second coat of latex paint to the walls. Unfortunately, it turned out that after a short period of time the NIBA diffused out, seemingly unhindered by the new paint layer. Thus, back-diffusion into air could not be effectively prevented in this way. One must, therefore, conclude that painted plasterboard walls act as a reversible sink for methamphetamine.
For other substances (e.g. formaldehyde), the adsorption/desorption behaviors of specific building materials are already well established [10]. Diffusion-impeding paints are also already on the market for rehabilitation of residences containing wood contaminated with pentachlorophenol, for example. However, these paints are hardly suitable for application on large surfaces, since they affect the humidity level of an interior space.
6.2 “Breaking Bad” in a Child’s Room
Additional work by Morrison and coworkers has shown that small children, in particular, are vulnerable to exposure to substances like methamphetamine in interior rooms. In this case, besides through inhalation, the potential for oral intake must be taken into account as well, which can represent a very significant exposure pathway.
With model calculations, using the assumption of a methamphetamine air concentration of 1 ppb, a likely distribution for the substance on upholstered furniture, clothing, and fabric-based toys was computed; from that, a possible daily uptake was estimated. For the oral pathway, calculations suggested a plausible daily ingestion of 60 μg of methamphetamine per kg of body weight and day. This value lies well above the reference dose of 0.3 μg/kg/d, and only slightly below a common dose for medical applications in the treatment of ADD (attention deficit hyperactivity disorder) [44]. A methamphetamine-containing drug of the latter sort available in the United States is marketed under the tradename Desoxyn®.
Also of interest are factors that could influence the accumulation of methamphetamine on surfaces. Parker and Morrison [45] have studied the effects of such substances as squalene, triglycerides, fatty acids, cholesterol, and cholesteryl esters, all of which are present in various skin oils and the lipid layer of human skin. At a methamphetamine air-concentration of 1 ppb, surface accumulations on surfaces are especially enhanced by fatty acids. Consequently, any surface subject to frequent touching could constitute a very effective sink for methamphetamines. The same applies to household dust, which often exhibits a high content of skin residues.
Morrison et al. [44] therefore recommend, even after a sanitation process, that one monitors the levels of methamphetamine in air, dust, and fabrics over an extended period of time. An obvious drawback with respect to this suggestion: one must first be aware that the residence in question once served as a meth lab!
We will continue in February with life on mars and man as a source of substances.
References
[37] T. Schripp et al., Does e-cigarette consumption cause passive vaping?, Indoor Air 2013, 23, 25–31. https://doi.org/10.1111/j.1600-0668.2012.00792.x
[38] S. Varughese et al., Effects of theatrical smokes and fogs on respiratory health in the entertainment industry, Am. J. Ind. Med. 2005, 47, 411–418. https://doi.org/10.1002/ajim.20151
[39] W. Schober et al., Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers, Int J. Hyg. Environ. Health 2014, 217, 628–637. https://doi.org/10.1016/j.ijheh.2013.11.003
[40] Umweltbundesamt, Stellungnahme der Innenraumhygienekommission (IRK) zu elektronischen Zigaretten (E-Zigaretten) (in German), Bundesgesundheitsblatt 2016, 59, 1660–1661. https://doi.org/10.1007/s00103-016-2464-y
[41] F. Harnisch et al., Die Chemie bei Breaking Bad, Chem. Unserer Zeit 2013, 47, 214–221. https://doi.org/10.1002/ciuz.201300612
[42] V. Kunalan at al., Clarification of route specific impurities found in methylamphetamine synthesised using the Birch method, Forensic Sci. Int. 2012, 223, 321–329. https://doi.org/10.1016/j.forsciint.2012.10.008
[43] D. Poppendieck et al., Desorption of a methamphetamine surrogate from wallboard under remediation conditions, Atmos. Environ. 2015, 106, 477–484. https://doi.org/10.1016/j.atmosenv.2014.09.073
[44] G. Morrison et al., Accumulation of gas-phase methamphetamine on clothing, toy fabrics, and skin oil, Indoor Air 2015, 25, 405–414. https://doi.org/10.1111/ina.12159
[45] K. Parker et al., Methamphetamine absorption by skin lipids: accumulated mass, partition coefficients, and the influence of fatty acids, Indoor Air 2016, 26, 634–641. https://doi.org/10.1111/ina.12229
The article has been published in German as:
- The Air that I Breathe – Der Innenraum als chemischer Reaktor,
Tunga Salthammer,
Chem. unserer Zeit 2017.
https://doi.org/10.1002/ciuz.201700779
and was translated by W. E. Russey.
The Air that I Breathe – Part 1
Chemistry of indoor air is a relatively new, diverse and interdisciplinary field surrounding us every day
The Air that I Breathe – Part 2
How dangerous are my household appliances?
The Air that I Breathe – Part 3
Emissions of e-cigarettes and other drugs
The Air that I Breathe – Part 4
Man as a source of substances, life on Mars, and what to learn from movies
The Air that I Breathe – Part 5
How does climate change affect indoor air quality?
See similar articles by Klaus Roth published in ChemViews Magazine





