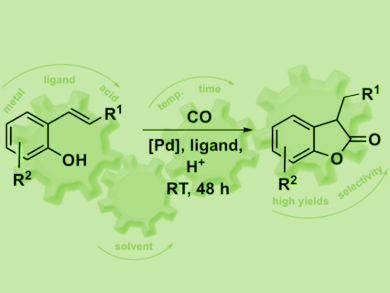
Benzofuranones are key structural elements of many bioactive and natural compounds. They are also used as antioxidants in polymer chemistry. The direct carbonylation of alkenols is a highly atom-economic approach for the synthesis of benzofuranones. However, these reactions are often performed under harsh reaction conditions and have a limited scope.
Ivana Fleischer, University of Tübingen, Germany, and Vera Hirschbeck, University of Regensburg, Germany, have developed a palladium-catalyzed intramolecular carbonylation of vinyl phenols. This reaction is carried out at room temperature using N-formylsaccharin (NFS) as a carbon monoxide surrogate to avoid the use of the toxic CO in a high-pressure reactor. The catalyst system consists of a Pd precursor, a bidentate phosphine ligand, and diphenylphosphoric acid (DPPA) as a co-catalyst.
The catalyst shows high activity for the carbonylation of various substrates, which was difficult to achieve before. For example, sterically hindered alkenes or those prone to polymerization at higher temperatures were successfully converted to the products in good yields and with high regioselectivity.
- Synthesis of Benzofuranones via Palladium-Catalyzed Intramolecular Alkoxycarbonylation of Alkenylphenols,
Vera Hirschbeck, Ivana Fleischer,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201705808

![Diazine-Tetraphenylethylene Cyclo[6]arenes for Molecular Recognition in Solution and Aggregate States](https://www.chemistryviews.org/wp-content/uploads/2025/11/ChemistryViews-2.70171-125x94.png)
