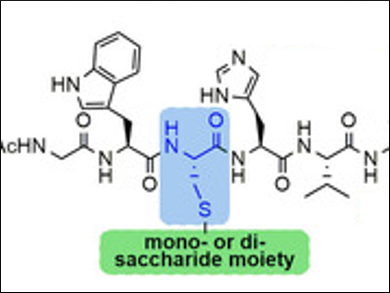
S-Glycosylated peptides have attracted considerable interest in drug synthesis and delivery. This is due to the fact that they are well tolerated by most biological systems and are characterized by an enhanced chemical stability and resistance to proteolytic enzymes.
Stefania De Luca, Institute of Biostructures and Bioimaging, Naples, Italy, and colleagues, have found an efficient and rapid synthesis for S-linked glycopeptides that uses very mild conditions. The synthesis relies on a cysteine residue which is inserted into a preformed peptide sequence as a sensitive substrate to bind carbohydrate derivatives through post‐synthetic S‐alkylation. The reaction occurs essentially through the SN2 reaction mechanism, leading to S‐β‐linked glycopeptides. The method uses only activated molecular sieves (MS) as the base and the appropriate glycosyl halide. The reaction takes place in N,N-dimethylformamide (DMF) and at room temperature.
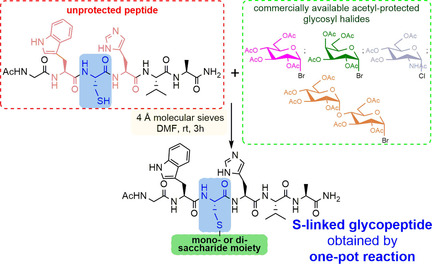
The sugar–peptide conjugates obtained from α-D-glycosyl derivatives adopt a β-S-configuration, indicating the high stereoselectivity of the substitution reaction. The peptide sequence does not require a specific activation of the thiol group to achieve the chemo- and stereoselective cysteine functionalization. Moreover, commercially available and relatively inexpensive acetyl-protected carbohydrate halides are used. This makes the method accessible to chemists who are not specialized in carbohydrate synthesis.
- Chemoselective Glycosylation of Peptides through S-Alkylation Reaction,
Enrica Calce, Giuseppe Digilio, Valeria Menchise, Michele Saviano, Stefania De Luca,
Chem. Eur. J. 2018, 24, 6231–6238.
https://doi.org/10.1002/chem.201800265