As you sip your coffee, ponder this: Oscar Francesconi, Stefano Roelens, University of Florence, Italy, and colleagues have developed two artificial receptors for the active component of your drink, caffeine. These receptors could be useful in biomedical and industrial applications.
Caffeine is a natural pesticide, an alkaloid found in parts of about sixty species of plant. Most well-known, of course, are the beans, or seeds, of the cultivated coffee plants Coffea arabica and Coffea canephora. As with many alkaloids, caffeine has a wide range of physiological actions. Most infamously, it is a stimulant of the central nervous system and this property has led to caffeine becoming one of the most widely used recreational drugs.
Connecting with Caffeine
Alongside caffeine, two related naturally occurring xanthine metabolites, theophylline and theobromine, are commonly found in a range of beverages and often used in conjunction with over-the-counter painkillers. This class of compounds has an inhibitory effect on adenosine receptors, stimulates the central nervous system, has a diuretic action, inhibits coughing, reduces bronchospasm, and perhaps even plays a role in slowing neurodegenerative disease.
The development of artificial receptors for caffeine has generally followed a biomimetic approach. The aim is to model the natural receptors in the laboratory for biomedical research, as well as for the development of sensors and other applications. A wide range of molecular structures have been successfully produced to “dock” with caffeine, including acyclic, macrobicyclic, clip, and tweezers-like structures, capsular structures, peptide-porphyrin complexes, triphenylene ketal scaffolds, and others. However, the team reports that although affinities ranging from milli- to micromolar values have been seen, the systems almost always suffer from a lack of selectivity.
Diaminocarbazolic Receptors
With the aim of finding better complexes for natural substrates, the team previously designed a macrocyclic receptor that is specific and capable of effectively recognizing monosaccharides. This artificial receptor with a shape-persistent cavity is based on diaminocarbazole units linked to anthracene moieties through methylene bridges. The very same structure might be readily adapted to build a receptor for caffeine and the other xanthine molecules, the team reasoned, given the shape and structure relationships between monosaccharides and the xanthenes.
Various tests were carried out with the original parent receptor structure and the team describes their success as “gratifying”, as it has an affinity for caffeine of 9.3 μM. According to the researchers, this is, to the best of their knowledge, “the largest value reported in the literature for an artificial receptor” for caffeine. However, within their error margins, the receptor was equally as receptive to theophylline. The selectivity to the individual xanthines for this structure is too limited to be of use in biomedical research.
Tweezers-Like Structure
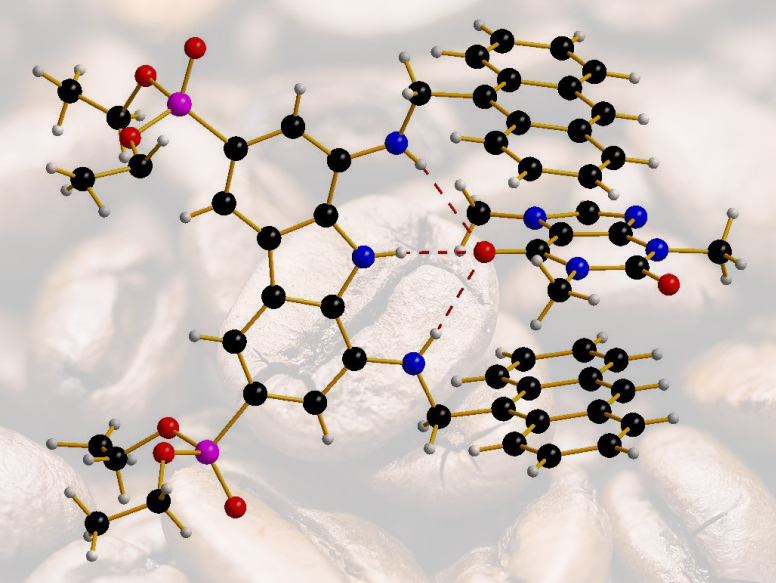
With the earlier success in hand, but in recognition of its limitations, the team then reasoned that an adaptive tweezers-shaped structure deprived of one of the two carbazole moieties might be more selective. With that modification, it could be able to discriminate between the trimethyl xanthine (caffeine, complex pictured) and the dimethyl xanthines (theophylline and theobromine). “Computational modeling in our work had a predictive role during receptor design,” Francesconi told ChemistryViews. He adds that “Experimental evidence of the actual complex is based on X-ray analysis, which shed light on the three-dimensional structure of the complex between the tweezers receptor and caffeine, revealing the origin of selectivity and the role of hydrogen bonding in the recognition.”
In the end, the team was able to demonstrate that the new tweezers-like structure has an affinity of 26 μM for caffeine and a 4.5-fold and 6-fold selectivity toward theophylline and theobromine, respectively. “The described structures represent a major advance in caffeine recognition,” the team states. “The next step in our work in progress concerns the study of caffeine recognition in water with water-soluble receptors, and preliminary results are very encouraging,” Francesconi explained.
Tiddo Mooibroek, an expert in supramolecular catalysis at The University of Amsterdam, The Netherlands, told ChemistryViews that “The work is very encouraging in that the team was able to finely tweak their architecture to rationally enhance the selectivity for their target—caffeine—while retaining micromolar affinity. I’m eager to read their next contribution about their water-soluble designs.”
- Effective Recognition of Caffeine by Diaminocarbazolic Receptors,
Oscar Francesconi, Andrea Ienco, Cristina Nativi, Stefano Roelens,
ChemPlusChem 2020.
https://doi.org/10.1002/cplu.202000114