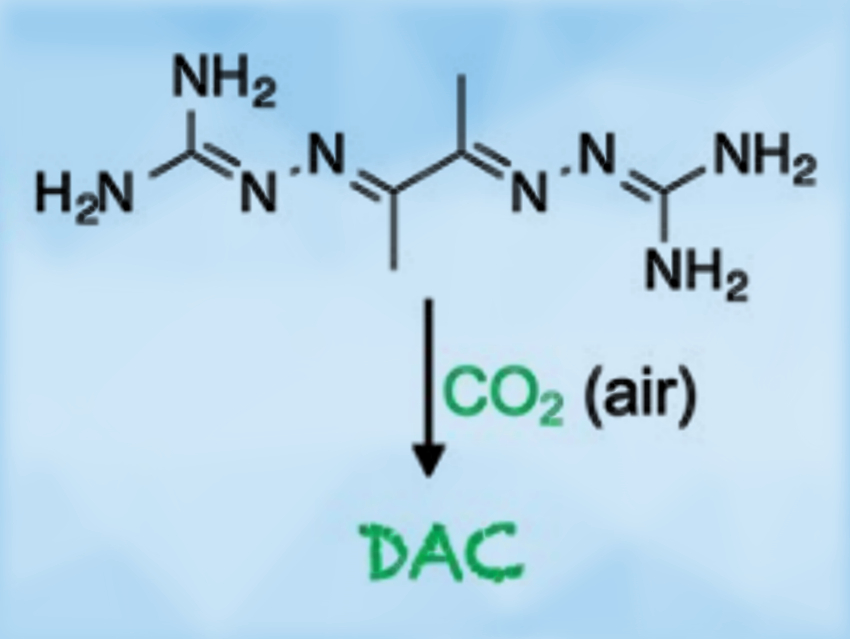
Direct air capture (DAC) technologies extract carbon dioxide from the atmosphere via chemical processes. They have the potential to remove past emissions and restore the atmospheric CO2 concentration to an optimal level.
Radu Custelcean and colleagues, Oak Ridge National Laboratory, TN, USA, have discovered an approach to DAC based on the crystallization of bis(iminoguanidine) (BIG) carbonate salts. BIGs (examples pictured below) are a class of anion-coordinating ligands. They are readily accessible via imine condensation of dialdehydes with aminoguanidinium salts. The CO2 absorption from air using these compounds is driven by the extremely low solubilities of BIG carbonate crystals in water.
The team studied a series of BIG structures resulting from glyoxal (GBIG, pictured below on the left) and its analogues methylglyoxal (MGBIG, pictured below in the middle) and diacetyl (DABIG, pictured below on the right). They found that minor modifications in the molecular structure of the BIGs result in major changes in the crystal structures and the aqueous solubilities within the series, leading to changed DAC properties.
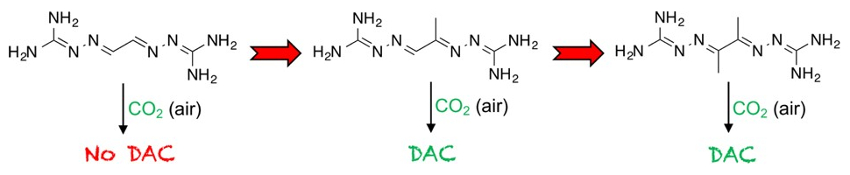
GBIG proved completely ineffective for DAC. Leaving an aqueous solution of GBIG open to air simply led to crystallization of the hydrated free ligand (GBIG ⋅ 2H2O). In contrast, MGBIG and DABIG can capture CO2 from air. The team attributes these differences to the absence of π‐stacking in MGBIG and DABIG and to the presence of stabilizing water molecules in the crystal structure.
- Dialing in Direct Air Capture of CO2 by Crystal Engineering of Bisiminoguanidines,
Radu Custelcean, Neil J. Williams, Xiaoping Wang, Kathleen A. Garrabrant, Halie J. Martin, Michelle K. Kidder, Alexander S. Ivanov, Vyacheslav S. Bryantsev,
ChemSusChem 2020.
https://doi.org/10.1002/cssc.202001114