α-Aminonitriles are highly useful and versatile intermediates for bioactive compounds such as alkaloids, as well as α-amino acids and 1,2-diamino compounds. The most common synthesis, the Strecker reaction, is a multistep synthesis and produces large amounts of waste. An alternative method uses environmentally benign oxidants with Ru, Fe, or vanadium-based catalysts, but suffers from poor recyclablitiy of the catalyst.
Bir Sain and co-workers, Indian Institute of Petroleum, Mohkampur, India, have synthesized a ruthenium-containing ionic liquid immobilized by expanded corn starch (ECS). The catalyst was applied to the oxidative cyanation of tertiary amines to α-aminonitriles (see scheme). It was shown to be a highly efficient and recyclable catalyst.
The biodegradable nature of the support, facile synthesis, efficient recycling, and high product selectivity, make this method an improved approach for this transformation.
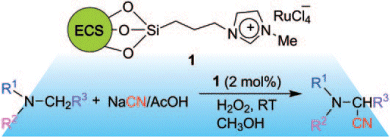
- Starch Immobilized Ruthenium Trichloride Catalyzed Oxidative Cyanation of Tertiary Amines with Hydrogen Peroxide
S. Verma, S. L. Jain, B. Sain,
ChemCatChem 2011.
DOI: 10.1002/cctc.201100111




