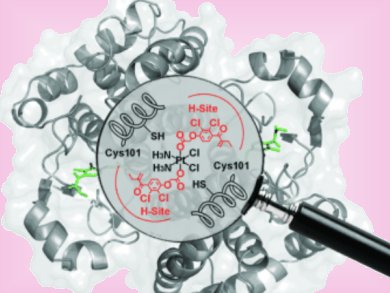
The effectiveness of platinum-based cancer drugs are limited by drug resistance, which, in some cancers, is associated to over expression of a major detoxifying enzyme; the π class glutathione S-transferase (GST P1-1). Ethacraplatin (EA-CPT) was designed as a cancer drug that could also target the GST enzymes.
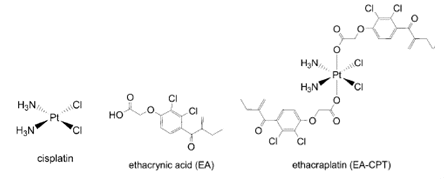
Michael Parker and co-workers, University of Melbourne, Australia, have combined X-ray crystallography and molecular modeling to visualize how EA-CPT is recognized by the dimeric GST P1-1 enzyme (protein backbone = pink ribbons) and why it is more potent than its individual components; ethacrynate acid (EA = orange spheres) and cisplatin.
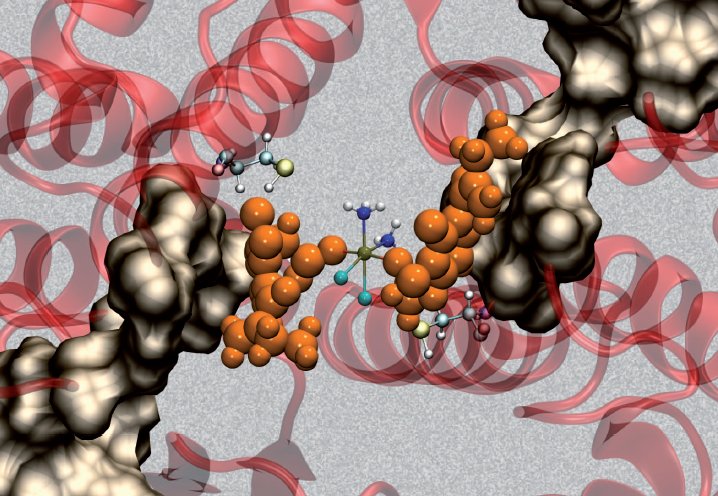
EA-CPT was shown to preferentially dock at the dimer interface at GST P1-1. Subsequent interaction with the enzyme resulted in docking of EA at both active sites, whereas the Pt moiety remained bound at the dimer interface. The EA ligands dissociate from the metal-bound enzyme complex, where they likely diffuse through a surface cavity (grey surface) a short distance to the active sites of each monomer, to covalently bind and inhibit the enzyme.
Images: (c) Wiley-VCH
- Studies of Glutathione Transferase P1-1 Bound to a Platinum(IV)-Based Anticancer Compound Reveal the Molecular Basis of its Activation
L. J. Parker, L. C. Italiano, C. J. Morton, N. C. Hancock, D. B. Ascher et al.,
Chem. Eur. J. 2011.
DOI: 10.1002/chem. 201100586