The chemical bonding in wheel-like planar boron clusters is remarkable; in addition to the strong and localized bonding in the circumferences, there are two types of delocalized bonding—the in-plane σ and the out-of-plane π bonding, each of which follows the (4N+2) Hückel rule for aromaticity.
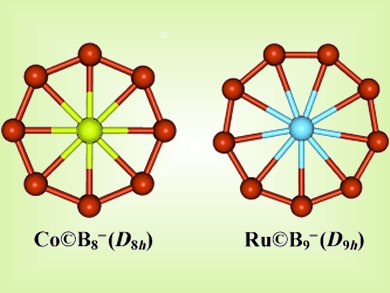
Lai-Sheng Wang and colleagues, Brown University, Rhode Island, USA, have made boron rings with a central metal atom. The Co©B8– and Ru©B9– clusters are unprecedented in chemistry due to their coordination numbers of 8 and 9 in perfect planar environments and the multiple aromaticity achieved by boron’s ability to form multicenter bonds
Because of the central position of the transition-metal atom, appropriate ligands may be conceived for coordination above and below the molecular plane, thus rendering chemical protection and allowing syntheses of this new class of novel boron-based metal complexes with unexpected chemical properties.
- Aromatic Metal-Centered Monocyclic Boron Rings: Co©B8− and Ru©B9−
C. Romanescu, T. R. Galeev, W.-L. Li, A. I. Boldyrev, L.-S. Wang,
Angew. Chem. Int. Ed. 2011.
DOI: 10.1002/anie.201104166