From Quasicrystals to Flexible Crystals
With crystals in the news thanks to the award of the 2011 Nobel Prize in Chemistry to Dan Shechtman for his 1982 discovery of quasicrystals, it is quite timely that I was writing about another crystalline discovery, this time from US chemist George Richter-Addo of the University of Oklahoma. His team’s discovery not only hints at the existence of a new type of “flexible” crystal, but also shows scientists how they might more easily probe such materials in greater detail.
Crystallographic studies of the coordination complexes of an important biochemical model compound have revealed intriguing new properties in crystals that allow closely packed molecules to have degrees of atomic movement closer to that seen in a liquid.
The biomolecules in question are the iron porphyrins. These structures are at the core of heme enzymes and many biomolecules but little is known about how oxygen donors coordinate to the iron atom despite this process being critical to the function proteins that bind to heme, such as catalases. Richter-Addo’s team have now taken a close look at how the diatomic and biologically important molecule nitric oxide (NO) coordinates axially to the iron center. Their findings not only add to our understanding of heme but have given rise to a puzzling new feature in crystals in which atoms in a solid crystal can move considerable distances without the structure breaking down. One might tentatively refer to such substances as flexible crystals.
Heme and NO
George Richter-Addo and colleagues Nan Xu and Douglas Powell were hoping to glean new insights into how the vasodilator nitric oxide works to inhibit heme catalase. They assumed and others have assumed that it does so by binding directly to the iron in the heme group. However, there have been no crystal structures reported for NO adducts of Tyr-ligated heme proteins and only two published showing how, surprisingly, NO might bind to model iron porphyrins with trans oxygen-donor ligands. This is surprising because in the late 1980s and early 1990s, scientists identified NO as a cellular signaling compound and key messenger in various biochemical processes in vertebrates. The molecule has many different roles particularly in smooth muscle relaxation, blood pressure control and sexual function.
The Oklahoma team is well known for its work over the last few decades on investigating the interactions of biological metals, including iron, with nitrogen oxides of environmental and physiological interest. Despite this, Richter-Addo concedes that it has been very difficult, almost impossible, to determine precisely the effects that nitric oxide has on some biological iron species. Part of the problem is that in laboratory preparations, the iron(III)-NO bond is relatively unstable and quickly reverts to an iron(II)-NO bond if there is an excess of NO.
Greater Detail of Heme Interactions
To preclude this from happening, Richter-Addo’s senior researcher Nan Xu has developed a new method using a solid-gas reaction to generate the required iron(III) by allowing gaseous NO to interact with the iron center and so controlling the rate at which coordination can occur and avoiding the excess NO. In an earlier study, this allowed the team to generate a heme nitrosyl thiolate compound. Now, they have used their unique solid-gas reaction to produce an iron(III)-NO model compound that forms suitable crystals for X-ray diffraction studies allowing a determination to be carried out that has been sought for decades, says Richter-Addo.
The model compound is produced from the precursor [(TPP)Fe(H2O)]SO3CF3 (where TPP is tetraphenylporphyrin) by the solid-gas reaction with NO and the team has obtained crystal structures of both the precursor and the NO-ligated product in the same crystals produced under the same reaction conditions simply by controlling the influx of NO gas. This approach might now allow researchers to study in great detail the way in which NO interacts with heme models and other related iron-containing biomolecules. Richter-Addo is very enthusiastic about the technique’s potential. “This is the first time that such results have been generated and published, and is sure to have a major impact in opening up new methodology all over the world,” he says.
Movement Within Crystals
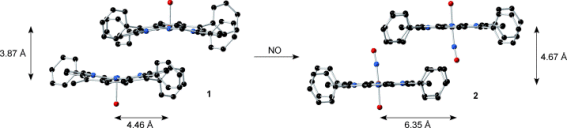
It is the substantial movements of molecules within the solid crystal as the reaction takes place that Richter-Addo believes is an even more compelling issue within the research. “Xu has shown that very large movements of molecules, up to 2 nm, are possible even in the solid crystals without the crystal structure fragmenting”, he explains, “This result will blow people away”.
The team’s X-ray structures show how diffusion of NO into the crystal lattice pushes the porphyrin rings apart so that adjacent oxygen atoms on the nitrosyl (NO) group, once ligated, are sufficiently far apart that one can assume that there is no interaction between them. The mean distance jumps from 0.388 nm to 0.467 nm in the precursor to the nitrosyl product, while there is also a lateral shift of the porphyrin rings in the crystal from 0.443 nm to 0.635 nm (pictured). The volume change is greater than the crystal unit cell, but nevertheless the solid-gas reaction does not lead to the crystal breaking apart.
“The success of the new crystal-gas methodology opens new doors for the isolation of unstable intermediates once considered impossible to obtain”, Richter-Addo told ChemViews magazine.
Tetrapyrrole expert and Chair of Organic Chemistry at Trinity College Dublin, Ireland, Mathias Senge, suggests that Richter-Addo’s work is “a very noteworthy contribution” and “certainly worth being highlighted”.
He suggests that any structural (porphyrin) chemist will immediately see the implications of this work. “It will serve as a route map for how to investigate small gaseous molecule interactions with coordination compounds”, he says. But, “more importantly this might reignite interest in solid state crystal chemistry in general”.
Images: © Wiley-VCH
- Nitrosylation in a Crystal: Remarkable Movements of Iron Porphyrins Upon Binding of Nitric Oxide
N. Xu, D. R. Powell, G. B. Richter-Addo,
Angew. Chem. Int. Ed. 2011.
DOI: 10.1002/anie.201103329