Unusual Class of Compounds
Persilastaffanes are an unusual new class of compounds that are introduced in the journal Angewandte Chemie by Japanese researchers led by Takeaki Iwamoto at Tohoku University. They are rod-shaped molecules with a core consisting of one or more tiny “cages” made of silicon atoms. Even more unusual than the name and structure of these materials are the properties of their electrons, which make the materials intriguing candidates as building blocks to make new materials for electronic applications.
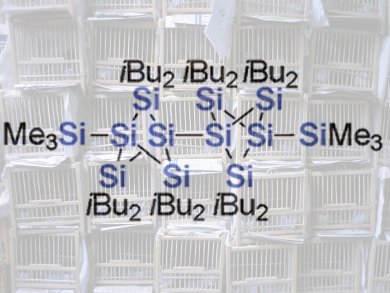
Where does the name persilastaffane come from? Persila indicates an organic molecule in which all (“per”) carbon atoms are replaced by silicon atoms (“sila”). A staffane is a special arrangement of five carbon atoms: two “bridgeheads” are bound to each other by way of three “bridges”, each of which has a carbon atom at its center. This results in a cage-like spatial structure. Alternatively, the structure of the cage can be viewed as a wavy ring made of four carbon atoms in which two opposite sides are additionally bridged by another carbon atom. A persilastaffane is a molecule that contains this type of cage made out of silicon atoms.
Delocalized Bonding
The Japanese team has developed a synthetic technique to make molecules containing one, two, or three such cages. What is so fascinating about these rod-shaped molecules? To date, there have been few studies of linear chains of silicon-containing ring systems; however theory suggests that there should be significant interactions between the cages. In these cases, the bonding electrons (sigma electrons) in the silicon–silicon bonds should not be localized between the two bonding partners as is usual in chemical bonds; instead, they should be able to move freely (delocalized) over the entire three-dimensional framework of silicon atoms, as in solid silicon.
This property is very interesting because silicon compounds with delocalized sigma electrons absorb light in the UV range, as well as being light-sensitive or conducting. They can also become conducting under light. Iwamoto and his colleagues examined the tiny rods by spectroscopic methods. They were able to confirm considerable delocalization of the sigma electrons over the silicon cages. Iwamoto remarks: “Persilstaffanes are fascinating rod-shaped silicon molecules that could serve as linear connectors for novel silicon-based finely defined materials, such as conductive molecular wires.”
- Persilastaffanes: Design, Synthesis, Structure, and Conjugation between Silicon Cages,
T. Iwamoto, D. Tsushima, E. Kwon, S. Ishida, H. Isobe,
Angew. Chem. Int. Ed. 2012.
DOI: 10.1002/anie.201106422