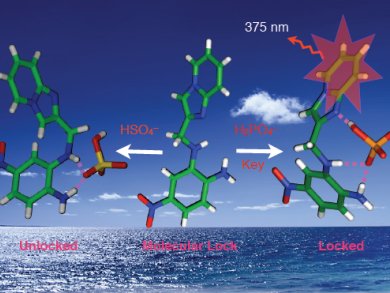
Phosphate ions play a crucial role in many physiological processes. Receptors that detect and signal the presence of such anions help us understand the roles they play in processes such as signal transduction and energy storage. Single-crystal X-ray analysis of phosphate-ion-bound receptors are very rare owing to the tetrahedral structure of the dihydrogenphosphate ion which creates problems for effective binding with receptors.
Akhtarul Alam, Aliah University, and Nikhil Guchhait, University of Calcutta, both India, and co-workers report a new acyclic receptor that acts as a “molecular lock” (pictured) upon anion binding. They describe a fluorophoric anion receptor with the imidazo[1,2-a]pyridine moiety having complementary hydrogen-bonding, donor-acceptor groups for efficient and selective recognition of the tetrahedral dihydrogenphosphate ion (“key”). The “key” efficiently freezes (“locks”) the conformational free rotation of receptor by hydrogen-bonding interactions, which was evidenced by single-crystal X-ray analysis. The restriction of free rotation results in enhancement of fluorescent intensity in CH3CN solution.
Structurally similar HSO4– did not act in a similar way and in the presence of other competitive anions (F–, AcO–, NO3–, ClO4–, Cl–, Br–, and I–) the receptor remained unchanged.
Image: © Wiley-VCH
- A Molecular Lock and Key: “Unlocked–Locked” Conformational Switching of a Receptor by Anions,
S. Dalapati, M. A. Alam, S. Jana, R. Saha, S. Biswas, N. Guchhait,
ChemPlusChem 2012.
DOI: 10.1002/cplu.201100036
This article is available for free as part of the ChemPlusChem free trial.