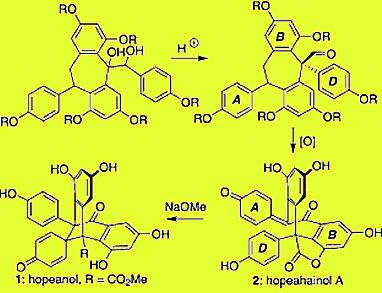
Scott A. Snyder and colleagues, Columbia University, New York, USA, describe a distinct approach for the total synthesis of the resveratrol dimers hopeanol and hopeahainol A empowered by a unique, reagent-driven pinacol rearrangement and substrate-specific oxidation chemistry.
Critical steps involved a pinacol rearrangement empowered by a chiral phosphoric acid and multistage, substrate-specific oxidation processes. Current efforts are directed towards developing asymmetric syntheses of these materials and probing their chemical biology.
The route has biogenetic implications that trace the origin of these compounds to more common dimeric family members.
- Total Syntheses of Hopeanol and Hopeahainol A Empowered by a Chiral Brønsted Acid Induced Pinacol Rearrangement,
Scott A. Snyder, Stephen B. Thomas, Agathe C. Mayer, and Steven P. Breazzano,
Angew. Chem. Int. Ed. 2012, 51.
DOI: 10.1002/anie.201107730

![Diazine-Tetraphenylethylene Cyclo[6]arenes for Molecular Recognition in Solution and Aggregate States](https://www.chemistryviews.org/wp-content/uploads/2025/11/ChemistryViews-2.70171-125x94.png)
