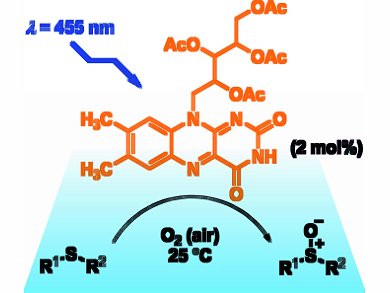
The oxidation of sulfides to sulfoxides, which are biologically active compounds in pharmaceuticals and important intermediates in organic synthesis, requires mild methods to avoid overoxidation to sulfones. Radek Cibulka and co-workers, Institute of Chemical Technology, Prague, Czech Republic, use flavins as efficient chemoselective sensitizers to photooxidize sulfides to sulfoxides.
Six 220 mW light-emitting diodes (LEDs) emitted blue light that irradiated the sulfide and tetra-O-acetylriboflavin catalyst. A range of alkyl, alkenyl, and aromatic sulfides, including sterically hindered substrates, were oxidized with quantitative conversions, molar yields up to 100 %, and quantum yields up to 0.7. The reaction proceeds under O2 at room temperature with low catalyst loading (2 mol %). The presence of water in the 95 % ethanol solvent increased the rate of photooxidation for several structurally different sulfides.
Flavins typically operate through an electron transfer mechanism, however, they are thought to mediate the photooxidation by a singlet oxygen mechanism. Structural modifications to improve the stability of the flavin catalyst are currently under investigation.
- Photooxidation of Sulfides to Sulfoxides Mediated by Tetra-O-Acetylriboflavin and Visible Light,
J. Dad’ová, E. Svobodová, M. Sikorski, B. König, R. Cibulka,
ChemCatChem 2012, 4, 620–623.
DOI: 10.1002/cctc.201100372