Chiral Catalysis Assisted by New Type of Hydrogen Bond
A novel type of hydrogen bond has been identified spectroscopically by researchers at ETH Zürich, Switzerland. The discovery could have implications for understanding platinum-catalyzed asymmetric hydrogenation and other reactions as well as raising the possibility of developing more efficient catalysts for this and related processes.
Fabian Meemken, Nobutaka Maeda, Konrad Hungerbühler, and Alfons Baiker have used in situ attenuated total reflection infrared spectroscopy (ATR-IR) together with modulation excitation spectroscopy (MES) to investigate the hypothetical O–H–O type hydrogen bonding that might occur between catalytic substrate and the chiral modifier in heterogeneous asymmetric hydrogenation on cinchona-modified platinum.
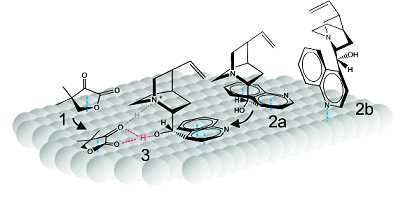
The O–H–O type hydrogen bond, which forms in the enantiodifferentiating diastereomeric surface complex between ketopantolactone and cinchonidine (Fig. 1, (3)), reveals a tie between the C9–OH of cinchonidine and the O=C of the substrate, C9–O⋅⋅⋅H⋅⋅⋅O=C. The team also observed a previously postulated N–H⋅⋅⋅O=C bond, which they suggest underpins the high enantioselectivity achieved with ketopantolactone on chirally modified platinum where a greater than 91.5 % enantiomeric excess is possible.
 © Wiley-VCH
© Wiley-VCH
Figure 1. Identified adsorbed molecules and their proposed interaction; 1 = ketopantolactone, 2a = π-bonded cinchonidine, 2b = N-lone pair bonded cinchonidine, 3 = ketopantolactone-cinchonidine complex.
But, why do we need to worry about yet another chiral catalyst? According to Baiker and colleagues, enantiopurity remains of crucial importance across the chemical industry from the manufacture of fine chemicals to pharmaceuticals and agrochemicals, flavors and fragrances. “The great demand and the limited natural resources of optically active compounds have enormously spurred research toward their economical and sustainable production,” they explain. As such, developing efficient strategies to make single-handed compounds is one of the most effective approaches to sustainability.
Combining Homo- and Heterogeneous Catalysis for Better Selectivities
Homogeneous asymmetric catalysis has made a big stir in this area, but heterogeneous asymmetric catalysis has lagged behind. That said, heterogeneous tools have the inherent advantage of easy separation simply because product and catalyst are in distinct phases rather than the same solution. One approach to combine the power of homogeneous chiral reagents with the benefits of heterogeneous catalysts is to adsorb appropriate chiral auxiliaries on to the solid catalyst surface. Of course, doing so effectively requires a clear chemical understanding not only of the adsorption process, but also what happens to the starting materials once those are added to the system.
Baiker and colleagues have found that adding just trace quantities of cinchona alkaloids to the reaction medium can boost the enantiomeric excess considerably in hydrogenation reactions of ketones on platinum catalysts. Such a standard reaction seems at first glance rather simple, but the reaction mechanism is far from that. By combining ATR-IR and MES, the team has now obtained important insights into the complexities of the solid-liquid interface in such catalytic reactions.
The team concludes that, “Our study provides the first in situ spectroscopic evidence for the possible role of the C9–OH functionality in cinchona alkaloids in the enantiodifferentiating diastereomeric complex formed between ketopantolactone and cinchonidine.” The revelation of a new type of hydrogen bond in this reaction will open up studies of related systems as well as helping chemists to improve catalyst design to boost those enantiomeric excesses still further.
- Platinum-Catalyzed Asymmetric Hydrogenation: Spectroscopic Evidence for O–H–O Hydrogen Bond Interaction between Substrate and Modifier,
F. Meemken, N. Maeda, K. Hungerbühler, A. Baiker,
Angew. Chem. Int. Ed. 2012.
DOI: 10.1002/anie.201203007
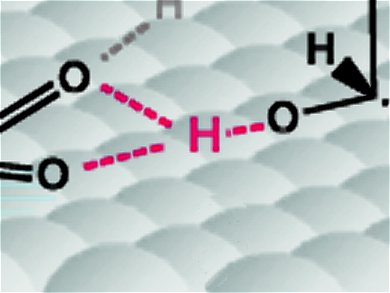




1. The figure of the above article contains one hydrogen which is bonded by three oxygen atom with hydrogen bond. Is it possible? Because, during the formation of hydrogen bond it is compulsory the formation of one sigma bond between hydrogen and more electronegative element (O, N, F) to develop polarity in the molecule, which is responsible for hydrogen bond formation. In the above figure – There are presence of one covalent bond and two hydrogen bond between oxygen and three hydrogen atom.
2. Theoretically hydrogen bond is represented by three DOT LINE , each dot is for each atom (O, N, F)
3. The figure is not match with the third para in which it contain ( O-H-O) type hydrogen bond bond.