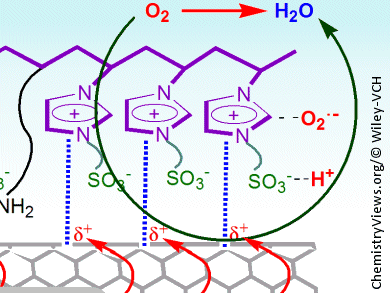
The oxygen reduction reaction is at the heart of many fuel cells and is usually catalyzed by platinum/carbon electrocatalysts. A team of researchers led by Chong-mok Lee and Sang-gi Lee, Ewha Womans University, Seoul, Korea, have attached imidazolium sulfonate zwitterions to multiwalled carbon nanotubes to make metal-free electrocatalysts that rival their commercial counterparts in activity and fuel selectivity.
The researchers first functionalized the carbon nanotubes with allyl imidazolium groups, which were then co-polymerized with vinyl imidazolium salts to give the electrocatalysts. Performance in the oxygen reduction reaction was evaluated using rotating disk electrode measurements and cyclic voltammetry. The results indicate that the imidazolium groups can polarize the nanotube π electrons, which leads to a positive charge on the nanotube surface. This polarization, along with the ability of the sulfonate group in the zwitterion to attract protons and keep them close to the electronically generated oxygen radical anion species, significantly increases the rate of the oxygen reduction reaction.
These findings show how the electronic structure of the imidazolium group influences the overall electrocatalytic performance of the system. Further optimization should lead to the development of a series of cost-effective metal-free catalysts for the oxygen reduction reaction.
- Insight into the Origin of the Positive Effects of Imidazolium Salt on Electrocatalytic Activity: Ionic Carbon Nanotubes as Metal-Free Electrocatalysts for Oxygen Reduction Reaction,
Youn Soo Kim, Ju Yeon Shin, Hyun Ji Jeon, Areum Cha, Chongmok Lee, Sang-gi Lee,
Chem. Asian J. 2012, 7.
DOI: 10.1002/asia.201200800