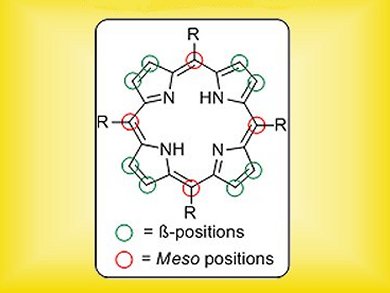
Porphyrins and their metal complexes have long been known to play a vital role in supporting life – the iron prohyrin in haemoglobin responsible for transporting oxygen in the blood is a classic example. In recent years, these macrocyclic compounds have been gaining increasing importance in diverse fields such as photodynamic therapy, functional materials and organic electronics. As a result, synthetic procedures that further functionalize the porphyrin core are highly prized.
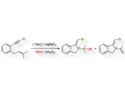
In 2011, Atsuhiro Osuka and co-workers, Kyoto University, Japan, reported a palladium-catalyzed approach for the bis-β-arylation of porphyrins. Now the team have shown that this method can also be applied to a porphyrin starting material bearing a sensitive formyl group. Mono and diarylation at the β-position(s) could be achieved by altering the reaction conditions leaving the formyl group available for further transformations. To exemplify this, the authors performed a McMurray reaction on compound A to form the porphyrin dimer B (see scheme).
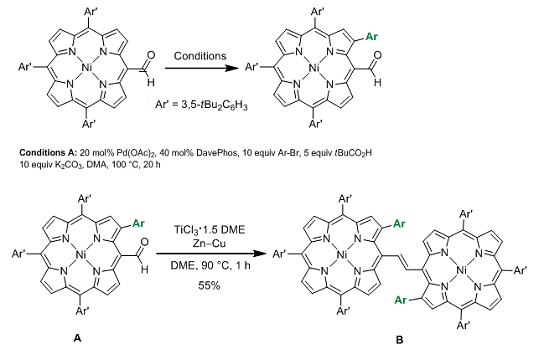
This added formyl group offers the opportunity to produce dimers with different tether lengths or containing additional functionality and there is potential to examine the effect of changing the nickel for another metal center. As a result, this opens up many new lines of investigation and potentially useful applications.
- Palladium-Catalyzed β-Selective Direct Arylation of Porphyrins,
Y. Kawamata, S. Tokuji, H. Yorimisu, A. Osuka,
Angew. Chem. Int. Ed. 2011, 50, 8867–8870.
DOI: 10.1002/anie.201102318 - Direct Arylation of meso-Formyl Porphyrin,
S. Tokuji, H. Awane, H. Yorimitsu, A. Osuka,
Chem. Eur. J. 2012.
DOI: 10.1002/chem.201203742