Difference Between p- and n-Type Electrodes
Photovoltaics continues to be an expensive technology. Dye-based solar cells may represent a more cost-effective alternative to traditional solar cells. In these cells, a dye is used in place of a semiconductor to trap the light. Tandem cells consisting of both a conventional n-type and an “inverse” p-type dye-sensitized solar cell seem to be especially promising. A team of Australian and German scientists has reported a significant increase in the degree of efficiency of p-type dye-sensitized solar cells through use of an electrolyte based on a cobalt complex.
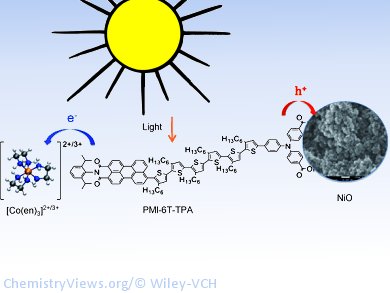
Conventional n-type dye-sensitized solar cells use a photoanode, a positive electrode coated with an n-type semiconductor, such as titanium dioxide, and a dye. When light strikes the electrode, the dye molecules become excited and release electrons—negative charges, hence the n in n-type—and “inject” them into the n-type semiconductor. The redox mediator, a component of the electrolyte that can move freely between the electrodes, regenerates the dye by resupplying it with electrons from the counter electrode. In a p-type cell, the process is reversed: a special dye and a p-type semiconductor are located on a photocathode. The light-activated dye “sucks” electrons out of the valence band of a p-type semiconductor such as nickel oxide. This effectively transfers “electron holes”—positive charges, hence the p in p-type—from the dye. The redox mediator takes the electrons from the dye and hands them over to the counter electrode.
Improving the Redox Mediator to Combine Electrodes
A very promising approach for increasing the performance of photovoltaic cells is to combine both an n-type and a p-type dye-sensitized solar cell to make a tandem cell. However, despite some progress, the performance of the p-type cells still significantly lags behind that of their n-type counterparts. An international team of researchers from Monash University and the Commonwealth Scientific and Industrial Research Organization, Australia, as well as the University of Ulm, Germany, have now achieved a considerable improvement in the efficiency of p-type cells by choosing a different redox mediator.
Researchers working with Udo Bach and Leone Spiccia replaced the previous, commonly used iodide and triiodide system with a well-known cobalt complex, tris(ethylenediamine)cobalt(II)/(III), in which the cobalt can switch between the +2 and +3 oxidation states. The advantage of this system is that the redox potential is significantly lower. As a result, the open-circuit voltage, a critical parameter for solar cells, is doubled and there is still a high enough driving force to ensure rapid and efficient regeneration of the spent dye. These devices achieve an energy-conversion efficiency of 1.3 %, while previous systems attained a maximum of only 0.41 %. The p-type dye-sensitized solar cell with the cobalt-based redox mediator even gave promising performance data under diffuse sunlight experienced on cloudy days.
Reference
- Highly Efficient p-Type Dye-Sensitized Solar Cells based on Tris(1,2-diaminoethane)Cobalt(II)/(III) Electrolytes,
Satvasheel Powar, Torben Daeneke, Michelle T. Ma, Dongchuan Fu, Noel W. Duffy, Günther Götz, Martin Weidelener, Amaresh Mishra, Peter Bäuerle, Leone Spiccia, Udo Bach,
Angew. Chem. Int. Ed. 2012.
DOI: 10.1002/anie.201206219