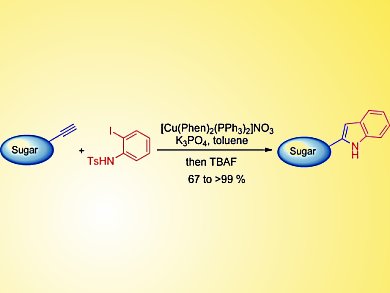
C-aryl glycosides which are an important class of C-glycosides comprise a large number of biologically active natural products that have an aromatic ring linked to the anomeric carbon of a sugar through a C–C bond. Several methods are available for the synthesis of 2-indole derivatives, but there are only a few reports on the synthesis of 2- and 3-indolyl-C-glycosides. These mostly involve the addition of N-protected lithio-indoles onto sugar-derived lactones or lactols followed by reduction or cyclization.
Parthasarathi Subramanian and Krishna P. Kaliappan, Indian Institute of Technology, Mumbai, have accomplished the synthesis of a variety of N-tosyl-2-indolyl-C-glycosides through a cascade reaction of C-alkynyl glycosides that involves a sequential Sonogashir type coupling and cyclative-hydroamination. They also extended this strategy to develop a one-pot synthesis of 2-indolyl-C-glycosides through tetrabutylammonium fluoride (TBAF)-mediated removal of the N-tosyl group. Notable features of this syntehsis include tolerance to sensitive functional groups and the selective functionalization of sugar-derived bis-alkyne.
The method serves as a straightforward alternative for the synthesis of 2-indolyl-C-glycosides and also facilitates the synthesis of related natural products. Further efforts to extend the scope of this methodology will include the total synthesis of α-C-mannosyl-tryptophan and its analogues.
- A One-Pot, Copper-Catalyzed Cascade Route to 2-Indolyl-C-glycosides,
Parthasarathi Subramanian Krishna P. Kaliappan,
Eur J. Org. Chem. 2013.
DOI: 10.1002/ejoc.201201208